Explain Distillation Of Water
Distillation operation which is performed at a pressure below atmospheric pressure is called as vacuum distillation. It is useful for producing water from salt solution.

Lab Science Can Be Fun Remember Doing This Just Also Remember Not To Drink The Wrong Alcohol Gcse Chemistry Distillation Process Distillation
This is performed by lowering the pressures in the column or.

Explain distillation of water. Water is the only component that is distilling. Solar water distillation is the process of using energy from the sunlight to separate freshwater from salts or other contaminants. Simple distillation is used to separate a solvent from a solution.
The sun supplies energy that causes water to evaporate from surface sources such as lakes oceans and streams. Distillation is a water purification process that uses a heat source to vaporize water and separate it from contaminants and other undesirable elements commonly found in ground and surface water. In other words distillation under reduced pressure is knows as Vacuum distillation.
The untreated water absorbs heat slowly reaching high temperatures. This can only happen according to Figure 1 if the temperature of the mixture is greater than 100 C. Fractional distillation is used in several industries like oil refineries and chemical plants mainly for purification and separation of many organic compounds.
Distillation is a widely used method for separating mixtures based on differences in the conditions required to change the phase of components of the mixture. Fractional distillation is also used for the separation of liquefied air. Water distillation is a water purification process that uses a heat source to vaporize water and separate it from the contaminants within.
To distill water all you really need is a heat source and a condenser. By collecting the water on a separate condenser the water has now gone through a process known as distillation. For example water can be separated from salt solution by simple distillation.
In both cases of rain evaporation and distillation the water leaves any harmful materials behind and rises. Distillation is perhaps the one water treatment technology that most completely reduces the widest range of drinking water contaminants. The same process can occur for mixed organic molecules where you generally get less complete separation with a single boiling cycle.
Distillation heats raw untreated water until the water reaches its boiling point and begins to vaporize. To separate a mixture of liquids the liquid can be heated to force components which have different boiling points into the gas phase. This method works because water has a much.
In nature this basic process is responsible for the water hydrologic cycle. Multistage flash distillation is a thermal process for desalting relatively large quantities of seawater. Simple distillation is a method for separating the solvent from a solution.
In nature the sun acts as a heat source to dissolve water after it rains. Fractional distillation is used for the purification of water as well as separating acetone and water. The cool air causes the water to then condense into droplets.
Based on the fact that the boiling temperature of water is lowered as air pressure drops this process is carried out in a series of closed tanks stages set at progressively lower pressures. The same process of condensation is at work during distillation which is essentially when a. Since the mole fraction of water in a mixture of sugar-water must be less than 1 in order for the observed vapor pressure of water to equal one atmosphere must be greater than one atmosphere.
A water distillation system is designed to purify water cheaply quickly and effectively. Most frequently the undesirable elements are what you find naturally in ground or surface water. Simple distillation works because the dissolved solute has a much higher.
Distillation works by applying heat to the water with solar distillation this is of course energy from the sun. The heat causes the water to evaporate cool and condense into vapour leaving the contaminants behind.

I Like To Think Of Myself As Up To Date About Skincare Ingredients And Products But Somehow Hydros Essential Oil Distiller Making Essential Oils Oil Distiller

The 3 Different Types Of Water Explained Distillation Distilled Water Best Water Filter

Organic Chemistry Purification Of Organic Compounds Askiitians Organic Chemistry Chemistry Practical Chemistry Classroom

Prepare Standard Solution 1 Solutions Preparation Distillation

Experimental Techniques Igcse Combined Science Teaching Resources Science Teaching Resources Science Science Resources

Desalination Drink A Cup Of Seawater Us Geological Survey Seawater Desalination Distillation Science Projects

How To Separate A Mixture Of Sugar Water Sugar Crystals Water Projects Mixtures
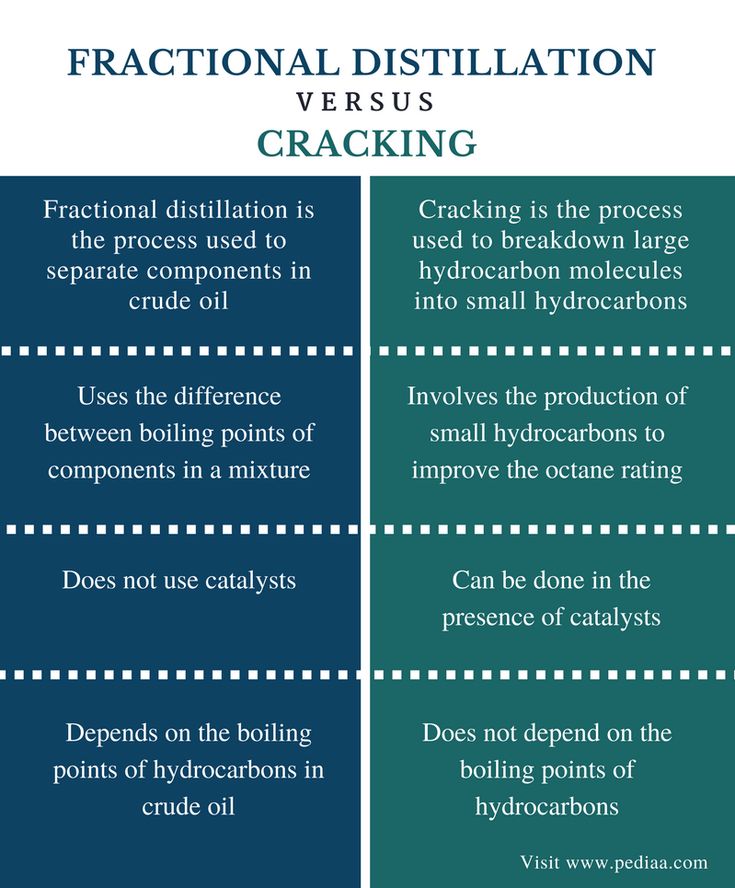
Difference Between Fractional Distillation And Cracking Comparison Summary In 2021 Fractional Distillation Distillation Basic Anatomy And Physiology

Distilling Water Distillation Distilled Water Water

Preparation Ethanol Laboratory Experiment 2 Alcohol What Is An Alcoholic Fractional Distillation

Distillation Process Of Chemicals Chemistry Experiments Chemistry Lab Equipment Chemistry

Diagram Of A Pot Still Pot Still Alcohol Still Society Of Wine Educators

Distillation Distillation Apparatus Distillation Chemistry

Distillation Pure Water Water Cooling

Ncert Exemplar Problems Class 9 Science Is Matter Around Us Pure Cbse Tuts Ncertsolutions Ncertsolut In 2021 Pure Products Chemistry Lessons Chemistry Education

What Is The Difference Between Reverse Osmosis Water And Distilled Water Reverse Osmosis Reverse Osmosis Water Osmosis

Fractional Distillation Distillation Distillation Process
Posting Komentar untuk "Explain Distillation Of Water"