Why Is Distilled Water A Neutral Substance
Handleys article about distilled water. It doesnt matter if the water comes out of your kitchen faucet is in a swimming pool or dog dish comes out of the ground or falls from the sky the water will contain significant amounts of dissolved substances minerals and chemicals.

Free Art Print Of The Ph Scale Universal Indicator Ph Color Chart Diagram Free Art Prints Art Prints Small Art Prints
A neutral substance such as pure distilled water has a pH of 7.

Why is distilled water a neutral substance. Distilled water is not a modern-day phenomenon but a benchmark of civilisation. Any solution with a pH under 7 is an acid and any solution with a pH over 7 is a base. In practice its difficult to put an exact number on the pH of distilled water because simple exposure to air can dissolve carbon dioxide.
This has a positive and direct effect on many processes in the body. In Aristotles Meteorologica where the philosopher discusses how seawater can be evaporated to produce drinking water. That means its neither an acid or a base.
Distilled water has a special property of being able to leach inorganic materials or toxic substances rejected by the cells and tissues and remove them from the body. The earliest recording of this substance is dated back to c. The pH of distilled water is theoretically about 7.
When you see distilled water H2O its a pure substance. It is the assumption that because distilled water has been purified it has a neutral pH of 7. Distilled water is a neutral substance.
A neutral substance such as pure distilled water has a pH of 7. The idea behind drinking distilled water to promote natural detoxification is that it doesnt flood your body with chlorine fluoride and other unpleasant compounds that can be in tap or bottled water. Water that isnt pure such as drinking water may contain other elements that can affect its acidity.
There may be many substances dissolved in the water and some of them may vaporize along with the water but salts and other solid solutes are left behind. Pure water is neutral because the total electric charges of the atoms and ions that compose it are equal and opposite so they cancel each other out. What Is Alkaline Water.
What is distilled water. If you want to decrease the pH of water you add an acidic substance such as lemon juice to it. Water is a most excellent solvent.
When you add pure water to an acidic solution it dilutes the mixture. Lets put the pH value of distilled water into context by discussing what distilled water is and why the pH of a substance is important. A mixture would be a glass of water with other things dissolved inside maybe one of those powders you take if you get sick.
384 322 BC. Because distilled water is void of minerals it is slightly acidic. Most parts of our body excluding things like stomach acid measure around 72 and 76 on the pH scale a 7 is neutral on the scale.
Does lime juice lower pH in water. Any solution with a pH under 7 is an acid and any solution with a pH over 7 is a base. But this is not always the case because distilled water is very rarely 100 pure and even more rarely has.
We can verify it by showing that neither blue nor red litmus paper changes its color when dipped in it. The acidity of a substance is measured on the pH scale. These things are the solutes dissolved in water.
Distilled water will be neutral. If you want to increase the pH of water you must add an alkaline substance such as baking powder to it. In addition to this distilled water is easier for cells to absorb and is more hydrating.
As far as acidity goes distilled water is close to a neutral pH and has no effect on the bodys acidbase balance. Adjusting pH in Water Pure or distilled water has a pH level of 7 which means it is neutral. This was distillation at its simplest.
Distilled Water Should Be pH Neutral The process for distillation involves boiling the water allowing the steam to condense in a tube and collecting the condensation in a container. Adjusting pH in Water Pure or distilled water has a pH level of 7 which means it is neutral. If you want to increase the pH of water you must add an alkaline substance.
Each of the substances in that glass keeps its own chemical properties. That means its neither an acid or a base. Distilled water is safe to drink and the kind of water I use myself Here is Dr.
Why does the pH of a substance matter. That means that there are only water molecules in the liquid. The pH scale which measures from 0 to 14 provides an indication of just how acidic or basic a substance is.

What Is The Ph Of Distilled Water The Chemistry Blog
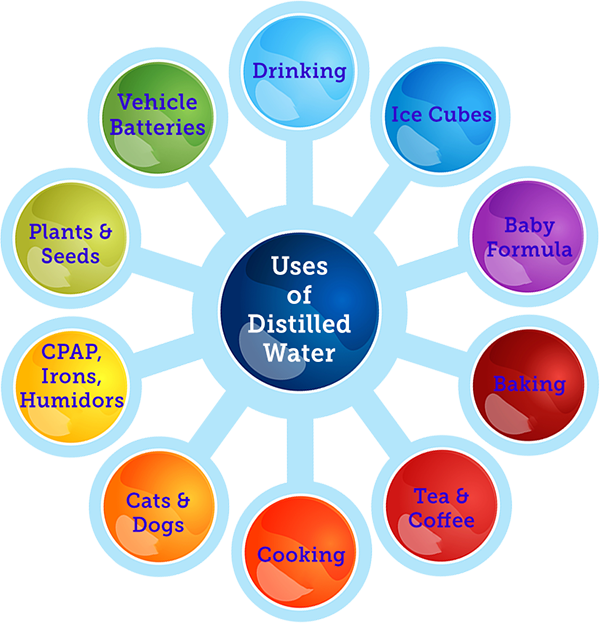
Uses For Distilled Water Aquanui Home Water Distillers

Simple Green Ph Scale Simple Green Yeast Infection Simple

5 Reasons To Never Drink Distilled Water Infographic Tyentusa

5 Reasons To Never Drink Distilled Water Infographic Tyentusa

Pin On Food Ahhhh Now I Get It

Skin Ph Matters Skins Ph Skin Care Serum Skin

5 Reasons To Never Drink Distilled Water Infographic Tyentusa

The Ph Scale Runs From 0 To 14 A Ph Of 7 Is Neutral Meaning The Two Types Of Ions Are In Balance So The Substa Neutral Meaning Distillation Alkaline Water

Food Ph Acidic Food Chart Acidic And Alkaline Foods Health And Nutrition

What Is The Difference Between Distilled Water And Deionized Water Di Water Page 2

Your Body Requires The Intake Of Minerals In Order To Remain Healthy Forever S Water Is A Source Of Natural Minerals In Coll Mineral Water Bottle Bottle Water
Distilled Water For Coffee Is It A Good Idea The Woke Lark




Posting Komentar untuk "Why Is Distilled Water A Neutral Substance"