Example Of Solution Homogeneous Mixture
The solute is dissolved in a solvent that is present in a larger quantity. When a homogeneous mixture contains only one phase then it is a solution.

Pin On Science Enviromental Studies
Salt water for example is a solution of solid NaCl in liquid water while air is a solution of a gaseous solute O 2 in a gaseous solvent N 2.

Example of solution homogeneous mixture. SOLUTIONS are homogeneous mixtures. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. The substance in the smallest amount and the one that dissolves or disperses is called the SOLUTE.
Sea water is a homogeneous solution having a higher percentage of salt and minerals than any other water source. A solution is a mixture of two or more substances in a single phase. Examples of homogeneous mixtures Wine.
That is then the product of completely dissolving a solute into a solvent without any undissolved particles. What is the difference between solution and homogeneous mixture. A list would go on for days and start with water.
Similarly rainwater is a homogeneous solution containing dissolved gases like O 2 CO 2 and some gases produced. Homogeneous mixtures are sources of water saline solution some alloys and bitumen. Homogeneous mixtures are also referred to as solutions.
In all cases however the overall phase of. A homogeneous solution tends to be identical no matter how you sample it. Therefore these are homogeneous solutions.
An example of a homogeneous mixture that is not a solution is homogenized milk. Which best describes a heterogeneous mixture. This type of mixture is also called the solution and is made by the solute and solvent.
The components of the solution are solute and solvent. The science of making wine and liquor is based on employing ethanol andor water as a solvent on various substances charred oak for bourbon whiskey for example or juniper in gin to create unique flavors. An example of a homogeneous solution is a soft drink.
A homogeneous solution would be water and alcohol or dissolved sugar or salt or sodium bicarbonate. The solutes are always present in low quantity. Homogeneous mixtures are sources of water saline solution some alloys and bitumen.
Of the choices below all are homogeneous mixtures except for a trail mix and c Italian dressing. At least two substances must be mixed in order to have a solution. Salt water for example is a solution of solid NaCl in liquid water while air is a solution of a gaseous solute O 2 in a gaseous solvent N 2.
A solution is a homogeneous mixture in which one or more substances the solutes are dissolved into another substance the solvent. Sand oil and water and chicken noodle soup are examples of heterogeneous mixtures. Wine - a homogeneous mixture like all liquors.
Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. Ie dissolving sugar in water cannot be separated. A homogeneous solution tends to be identical no matter how you sample it.
Solutions exist for every possible phase of the solute and the solvent. Heterogeneous mixtures are not mixed evenly so they do not appear uniform. In all cases however the overall phase of.
Solutions exist for every possible phase of the solute and the solvent. A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. Sand oil and water and chicken noodle soup are examples of heterogeneous mixtures.
That makes it one phase because no solid is tangible or visible. A solution is a homogeneous mixture for example lemonade is a solution of lemon and water. Most of the time it is a liquid while the solvents are present in the dominant quantity.
While we normally think of solutions as liquids such as soft drinks and lemonade they can actually be. Homogeneous Mixtures are those in which participants can not be distinguished and also cant be identified. Yes a solution is a homogeneous mixture.
Vinegar and window cleaner are homogeneous mixtures. Examples of heterogeneous mixtures include pizza and peanut butter and jelly sandwiches. Is milk a homogeneous mixture.
This substance which contains water sugar yeast and fruits that are mixed uniformly is another example of homogeneous mixtures.
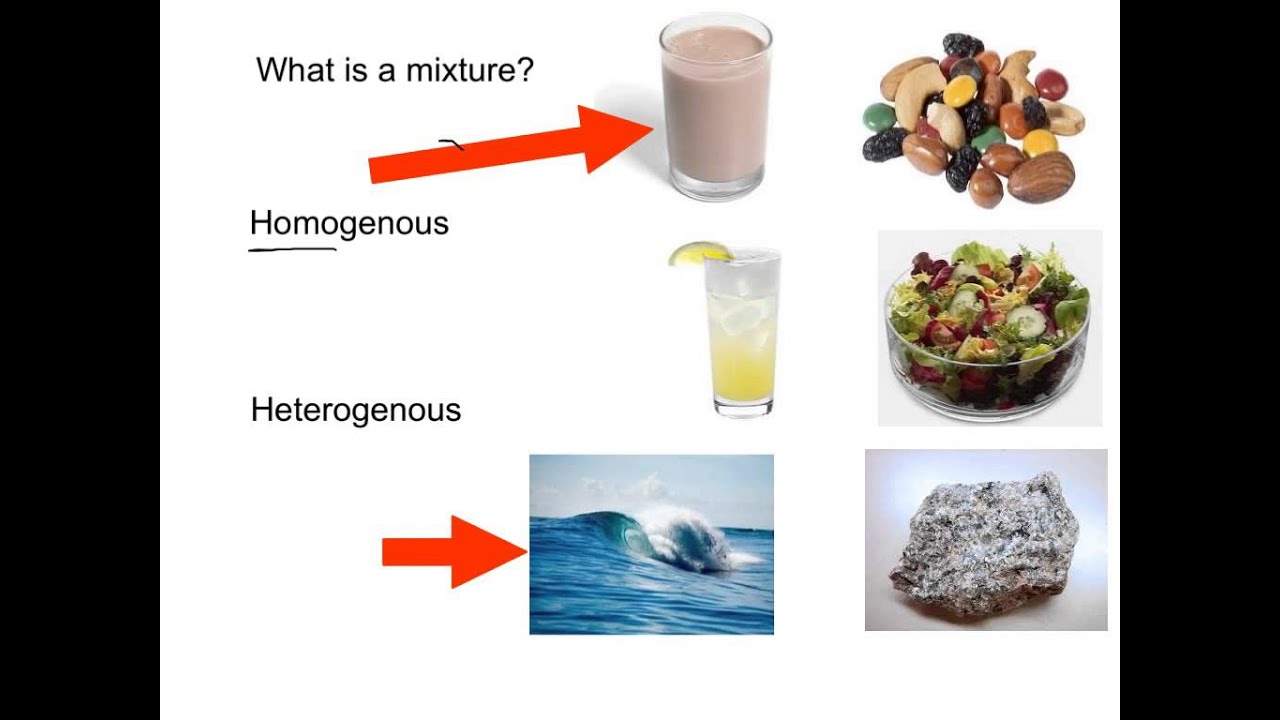
Pin On Middle School Chemistry

Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture

Mixtures And Pure Substances Editable Powerpoint And Notes Solutions And Mixtures Powerpoint Editable Powerpoint

Heterogeneous And Homogeneous Mixtures Worksheet Middle School Science Experiments Elementary Science Experiments Science Experiments For Preschoolers

Heterogeneous And Homogeneous Mixtures What S The Difference Examples Of Mixtures Homogeneous Mixture Heterogeneous Mixture

Let S Do An Experiment Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture Solutions And Mixtures 5th Grade Science

Pure Substances And Mixtures Homogeneous Mixture Heterogeneous Mixture Elements Compounds And Mixtures

Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Matter Worksheets

Chemistry For Kids Solutions And Dissolving Chemistry For Kids Solutions And Mixtures Elements Compounds And Mixtures

Homogeneous And Heterogeneous Mixture Heterogeneous Mixture Homogeneous Mixture Mixtures

Solution Suspension And Colloid Ppt Google Search Teaching Chemistry High School Science Chemistry

Homogenous Homogeneous Mixture Heterogeneous Mixture Elements Compounds And Mixtures

Mixtures Homogeneous Mixture Solutions

Mixtures And Solutions 5th Grade Science Physical Science Lessons Science Journal

Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards

What S The Difference Between Heterogeneous And Homogeneous Mixtures Examples Of Mixtures Mixtures Homogeneous Mixture

Heterogeneous Mixture Heterogeneous Mixture Easy Science Mixtures
Posting Komentar untuk "Example Of Solution Homogeneous Mixture"