Copper Aluminum Voltaic Cell
The two electrodes are connected by. When the top and bottom contacts were connected by a wire an electric.
Chemistry 30 Electrochemistry Calculating Voltage Of Electrochemical Cells
If aluminum is oxidized the potential is 166 electron volts.
Copper aluminum voltaic cell. Click card to see definition. A voltaic cell is a device which converts chemical energy to electrical energy. AlS I AI310 M II Cu210 MI CuS Which of the following statements is false.
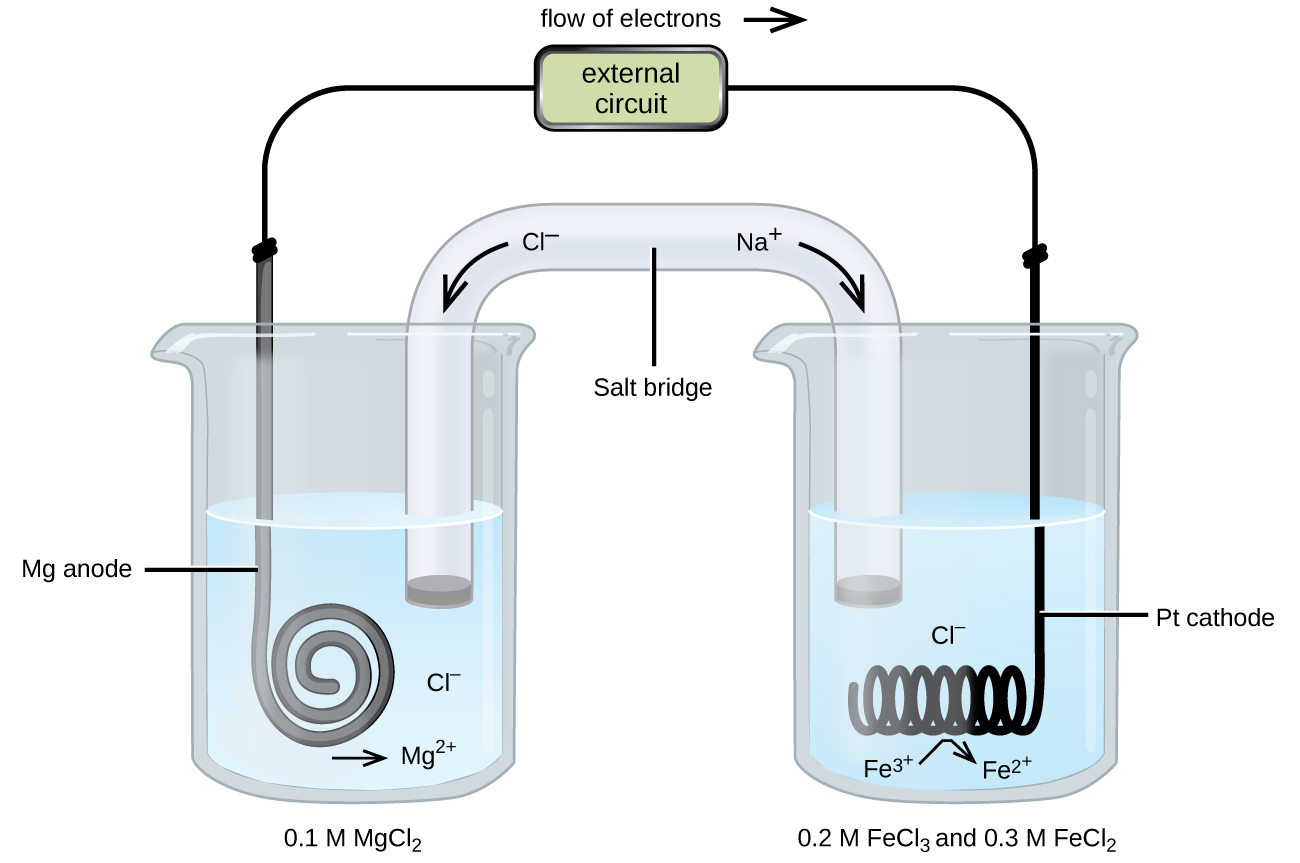
In a voltaic cell the oxidation and reduction of metals occurs at the electrodes. A voltaic cell is a type of electrochemical cell ie in these type of cells electrical energy is produced when a chemical change occurs. Through electrochemistry these reactions are reacting upon metal surfaces or electrodes.
A schematic of the Al-H cell is shown to the right. Als Al3aq Cu2aq Cus Which of the following reactions occurs at the anode. The copper electrode is the anode O e.
A voltaic cell prepared using aluminum and copper has the following cell notation. A postage balance is used to measure the mass of two copper electrodes prior to the demonstration. See the answer Consider a aluminum-silver voltaic cell that is constructed such that one half-cell consists of the aluminum Al electrode immersed in a Al NO33 solution and the other half-cell consists of the silver Ag electrode immersed in a AgNO3 solution.
Electrons flow through the external circuit from the aluminum electrode to the copper electrode A aluminum -copper voltaic cell is represented as follows. In this experiment you will be setting up ___________. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
The top of one Cu electrode is connected to the negative terminal of a DC. Each cell consists of a 2p coin a piece of blotting paper soaked in vinegar and a zinc disk. In Part I of this experiment you will use a voltmeter to measure the potential of a voltaic cell with copper.
How does a voltaic cell work. There are two electrodes in a voltaic cell one in each half-cell. The cathode is where reduction takes place and oxidation takes place at the anode.
A simple voltaic cell is made by placing two different metals in contact with an electrolyteThe metals act as the electrodes for the voltaic cell. Repeat the steps and create another cell starting with another copper coin. This pile of 10 cells had a measured voltage of 97v clearly not all the cells are at 102v.
English chemist John Frederick Daniell developed a voltaic cellin 1836 which used zinc and copper and solutions of their ions. The balanced chemical equation is as follows. Two requirements of an electrolytic cell are that a redox reaction occur and that the potential be negative.
Each cell gave roughly 102v apparently zinc-copper should be 11v at 25C and then its just a question of making a pile of them to make a larger voltage. Place a zinc washer on the cotton pad should be a similar size to the coin. In 1800 Volta stacked several pairs of alternating copperor silver and zincdiscs electrodes separated by cloth or cardboard soaked in brine electrolyte to increase the total electromotive force.
To illustrate the basic principles of a galvanic cell lets consider the reaction of metallic zinc with cupric ion Cu 2 to give copper metal and Zn 2 ion. The electrodes are placed in 10 M CuSO 4 aq solution. The two solutions are connected by a salt bridge eg an agar suspension of potassium nitrate.
Tap again to see term. When your voltaic cell is set up and has positive value for the cell potential the black connector wire is attached to the anode the half-cell in which oxidation is occurring. Click again to see term.
If copper is oxidized. You should now have a one cell copperelectrolytezinc. Voltaic Galvanic Cells.
Place multimeter on aluminium connector and on the zinc and you should get a voltage reading of around 05V. The chemical reactions that take place inside the cell cause the flow of electrons and hence electricity is produced. Consider the voltaic cell that is constructed by connecting the standard hydrogen electrode SHE to a second half-cell consisting of an aluminum electrode submerged in a 1 M Al3aq solution.
D none of the above.
Voltaic Cells Chemistry For Non Majors
How To Make A Battery At Home Out Of Table Salt And Aluminum Electrochemical Cell Energy Technology Battery
Http Drsapnag Manusadventures Com Chemistry General Chemistry Generalpowerpoint Pp19 02electrochememf Pdf
20 4 Cell Potential Under Standard Conditions Chemistry Libretexts
A Voltaic Cell Prepared Using Aluminum And Copper Has The Following Cell Notation Al S Al3 Aq Cu2 Aq Cu S Which Of The Following Reactions Occurs At The Anode A Al S Rightarrow
Galvanic Cell With Zinc And Copper Galvanic Cell Chemistry Lessons Electrochemistry
5 2 Galvanic Cells Chemistry Libretexts
4 Ways To Make A Homemade Battery Battery Homemade Conductive Materials
Galvanic Cells Chemistry For Majors
Simple Voltaic Cells Batteries Copper Zinc Cell Gcse Chemistry Ks4 Science Igcse O Level Revision Notes
Galvanic Cell Animation Requires Flash Physique Chimie Chimie
Electrochemical Cells Ck 12 Foundation
11 7 Electrolysis Chemistry Libretexts
Electrochemistry Chemistry For Non Majors
Electrochemical Cells Ck 12 Foundation
Why Is Mass Gained At The Cathode Chemistry Stack Exchange
Posting Komentar untuk "Copper Aluminum Voltaic Cell"