Enthalpy Of Solvation Table
Solute solvent solution Heat of solution or enthalpy of solution is the energy released or absorbed when the solute dissolves in the solvent. Many other properties can be found in.

As Table 3 For Cyclohexane Cyclohexanol And 1 2 Cyclohexanediol Download Table
It depends on the final concentration of the solute.

Enthalpy of solvation table. It can be considered as enthalpy of solvation with the solvent being water. It explains how to calculate the enthalpy o. Water is considered to be a polar solvent because it has a positive H atom and negative O atom poles.
For some special solutions. Salt-water sugar-water alcohol-water hydrogen peroxide-water ammonia-water and carbon dioxide-water. This chemistry video tutorial provides a basic introduction into enthalpy of solution and enthalpy of hydration.
This relates the enthalpy of solution to ion solvation enthalpies and to lattice enthalpy. Released 57 kJmol of heat of solution. FARADAY TRANS 1991 8718 2995-2999 2995 Thermodynamics of Solvation of Ions Part 54ibbs Free Energy of Hydration at 29815 Kt Yizhak Marcus Department of Inorganic and Analytical Chemistry The Hebrew University of Jerusalem Jerusalem.
PHYSICAL AND CHEMICAL DATA 2-3 2-146 Linear Expansion of Miscellaneous Substances. Here is the enthalpy transfer of reagents 1 and 2 from the reference solvent S o to other one and and are the enthalpies of activation of reaction in these solvents 12 13. The ΔH soln values given previously and in Table 822 for example were obtained by measuring the enthalpy changes at various concentrations and extrapolating the data to infinite dilution.
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements with all substances in their standard statesThe standard pressure value p 10 5 Pa 100 kPa 1 bar is recommended by IUPAC although prior to 1982 the value 100 atm 101325 kPa was used. As an example the relative changes of the enthalpy of solvation of a transition state can be calculated corresponding to the following. For a chemical reaction M g aq M aq Enthalpy change H Hyd.
Formula State H f 0 S0 G f 0 Am 2O 3 s 175728 15472 167778 AmO 2 s 100500 8368 95019 Ar g 000 15473 000 As s alphagray 000 3515 000 As 2 g 22217 23932 17196 As 2O 5 s 92487 10544 78241 As 2S 3 s 16903 16359 16862 As 4 g 14393 31380 9247 As 4O 6 s octahedral 131394 21422 115252 As 4O 6 s monoclinic 130959 23430. Using these data 100LC 8H 18 1000mLC 8H 181LC 8H 18 0692gC 8H 181mLC 8H 18 1molC 8H 18114gC 8H 18 5460kJ 1molC 8H 18 331 104kJ. Tables 411 and 412 show that solvation enthalpies for a cation such as K or Ba 2 tend to be of the same order of magnitude though strong donor solvents such as DMSO or HMPA do give markedly more favourable solvation enthalpies.
The amount of heat released or absorbed when a substance is dissolved is not a constant. Absolute Enthalpies of Hydration of Gaseous Ions. 2-130 2-147 Cubical Expansion of Liquids.
Because ΔH soln depends on the concentration of the. 3 m is the mass mass of the reactants mass of water mass of calorimeter C is the. The enthalpy of combustion of isooctane provides one of the necessary conversions.
The solution including the reactants and the products and the calorimeter itself do not undergo a physical or chemical change so we need to use the expression for specific heat capacity to relate their change in temperature to the amount of heat q cal that they have exchanged Eqn. Standard Enthalpy of Formation for Atomic and Molecular Ions Cations ΔH f kJmol Cations ΔH f kJmol Anions ΔH f kJmol Anions ΔH f kJmol Agaq 1059 Kaq 2512 Braq 1209 H 2PO 4 aq 13025 Al3aq 5247 Liaq 2785 Claq 1674 HPO 4 2aq 12987 Ba2aq 5384 Mg2aq 4620 ClO. Table 671 gives this value as 5460 kJ per 1 mole of isooctane C 8 H 18.
Molar heat of solution or molar enthalpy of solution is the energy released or absorbed per mole of solute being dissolved in solvent. Heat of solution data. 16 rows Character Tables.
However for Ag solvation by DMSO MeCN and ammonia is particularly favourable. Invariably the enthalpy of solution of a salt is the small difference between the large enthalpy needed to separate the ions from each other in the crystal lattice and the enthalpy gained when. Hydration enthalpy is also called hydration energy and its values are always negative.

Pdf Solvation Enthalpies And Gibbs Energies Of The Proton And Electron Influence Of Solvation Models Semantic Scholar

Thermodynamic Functions Of Solubility And Solvation Processes Of Download Table

And Solvation Enthalpies Of Aromatic Amines In Benzene And Their Download Scientific Diagram

Table 1 From Ionic Hydrogen Bond Networks And Ion Solvation 1 An Efficient Monte Carlo Quantum Mechanical Method For Structural Search And Energy Computations Ammonium Water Semantic Scholar

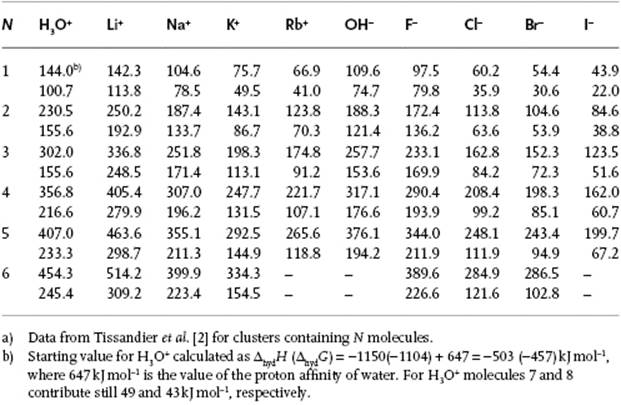
Table 1 From The Proton S Absolute Aqueous Enthalpy And Gibbs Free Energy Of Solvation From Cluster Ion Solvation Data Semantic Scholar
Hydration Structure Mixing Liquids Ionic Solutions Liquid State Physical Chemistry Fundamentals Modeling And Applications 2013

Enthalpy And Free Energy Changes Caused By Cyclohexane Solvation Download Table

Standard Molar Enthalpies Of Formation And Vaporization Of Dmbua Download Table

Enthalpies Of Cavity Formation And Changes Of Soll Uteesolvent Download Table

Table 4 From The Proton S Absolute Aqueous Enthalpy And Gibbs Free Energy Of Solvation From Cluster Ion Solvation Data Semantic Scholar

Standard Values Of Enthalpies Of Dissolution D Sol H Kj Mol Of Download Table

Standard Values Of Enthalpies Of Dissolution D Sol H Kj Mol Of Download Table

Table 1 From The Proton S Absolute Aqueous Enthalpy And Gibbs Free Energy Of Solvation From Cluster Ion Solvation Data Semantic Scholar

Pdf Solvation Enthalpies And Gibbs Energies Of The Proton And Electron Influence Of Solvation Models Semantic Scholar

Enthalpies Of Solution Sublimation And Estimated Enthalpy Of Download Table

Enthalpies Of Solution Sublimation And Estimated Enthalpy Of Download Table

Enthalpies Of Solvation Kcal Mol 1 For Alkanes In Water Fmd And Dmso Download Table
Posting Komentar untuk "Enthalpy Of Solvation Table"