Unavoidable Errors In Chemistry Labs
A digital balance showing three decimal places can only weigh to. Some of them are typical human errors that can be limited by sticking to lab procedures but as long as there is a human operator involved they will be never completely.
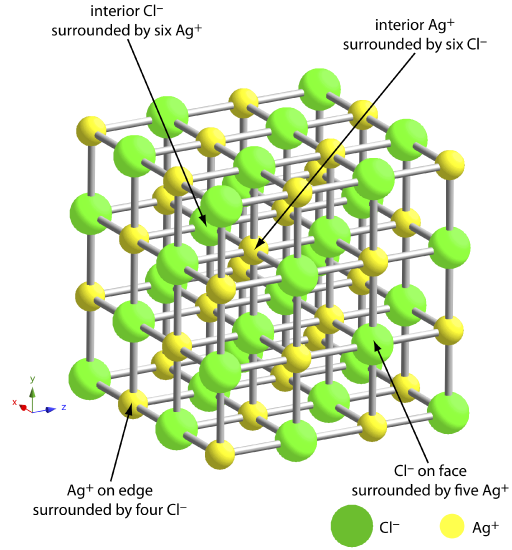
8 2 Precipitation Gravimetry Chemistry Libretexts
Uncertainties are inherent in any measuring instrument.

Unavoidable errors in chemistry labs. Lab ReportTypes of Experimental Errors. Such errors are always present in an experiment and largely unavoidable. When I reviewed the lab with students when I passed it back I made sure to discuss the difference between avoidable and unavoidable errors with examples from this lab.
Poor lab technique not reading the meniscus at the bottom at eye level. Finally there are thousands of possible random errors that cant be adjusted for. This is unavoidable RANDOM ERROR and does not reflect on your lab technique.
Forgetting to zero the scale. Experimental Errors - errors due equipment or. Unlike random errors these errors are always in the same direction.
Unacceptable its assumed that the results will be discarded and the experiment redone. Also using large 20 or 25 mL single volume pipettes means smaller relative errors. A ruler even if as well-made as is technologically possible has calibrations of finite width.
I have posted the lab description and the questions exact wording. A student generates graph of 1λ as a function of 1n 2 that yields a line characterized by the equation y-11 x. Errors for Finding the ratio of moles of reactants in a chemical reaction lab incorrect volume and temperature measurements not looking at the bottom of the meniscus for these inconsistent stirring lid not always on thermometer in the air instead of liquid.
WUBOLAB laboratory glassware has been committed to becoming a professional company that provides quality lab glassware to our customers worldwideWe have two factories to manufacture laboratory glassware and have more than 15 years experience in glassware productionWUBOLAB team committed to be our customers trust lan assitantlaboratory glassware. In chemistry accuracy refers to how close a measurement is to its standard or known value. What Color Is the Hottest Flame and What Do Different Colors Mean.
What are some unavoidable inherent errors in an extraction lab. Some examples of unavoidable errors are the precision limits of the balance and the graduated cylinder. If you answer clearly and thoroughly I will rate you The picture that is missing says thisCritically evaluate your results and give resonable hypotheses that explain how the above values differ from the ideal.
Measuring the mass of a sample on an analytical balance may produce different values as air currents affect the balance or as water enters and leaves the specimen. Students are often inclined to cite user error as a main cause for a loss of yield in any process. Using This Checklist Use this checklist as a preliminary guideline when thinking about and analyzing potential errors in your experiment.
The absolute uncertainty expresses the margin of uncertainty associated with a reading a measurement or a calculation involving several readings. Such errors are always present in an experiment and largely unavoidable. Allowing the thermometer to touch the glassware etc.
Post-Lab 2 Experiment 2 Post Lab 5 - Post lab CHM 2210 Syllabus Orgo Lab 6 - Organic lab PCB3063 Chapter 03 - Genetics Other related documents DOandIOPracticeAnswers Experiment 24 HSB Review Chapter 3 Quiz Solutions Aspirin Synthesis Lab Report ReactionGuideforExam3. A 250 cm 3 pipette of grade B accuracy delivers this volume to within 006 cm 3 if used correctly. When weighing yourself on a scale you position yourself slightly differently each time.
It is common for new organic chemistry students to be disappointed by a low yield anything less than 95 and to worry that it is somehow their fault. The list is a guide but is not comprehensive so make sure that you check with your instructor about the different types of errors to pay attention to in your lab. The table given below lists the absolute uncertainties for some equipment used in the Chemistry lab.
In chemistry the same is true when we talk about precision of measurementsPrecision refers to how close two or more measurements are to each other regardless of. Know how to use R h to calculate wavelength resulting from an energy transition. Random errors occur as a result of sudden random changes in an experiments conditions.
A mis-calibrated balance will always give results that are too high or too low depending on the direction of mis-calibration. Know how to calculate the energy required to ionize the electron from an atom Based on data in your lab notebook or the graph you generated sketch a graph of graph of 1λ as a function of 1n 2. When taking a volume reading in a flask you may read the value from a different angle each time.
The systematic errors are caused by the way we did the experiment.

Polymers Free Full Text Removal Of Cd2 From Water By Use Of Super Macroporous Cryogels And Comparison To Commercial Adsorbents Html
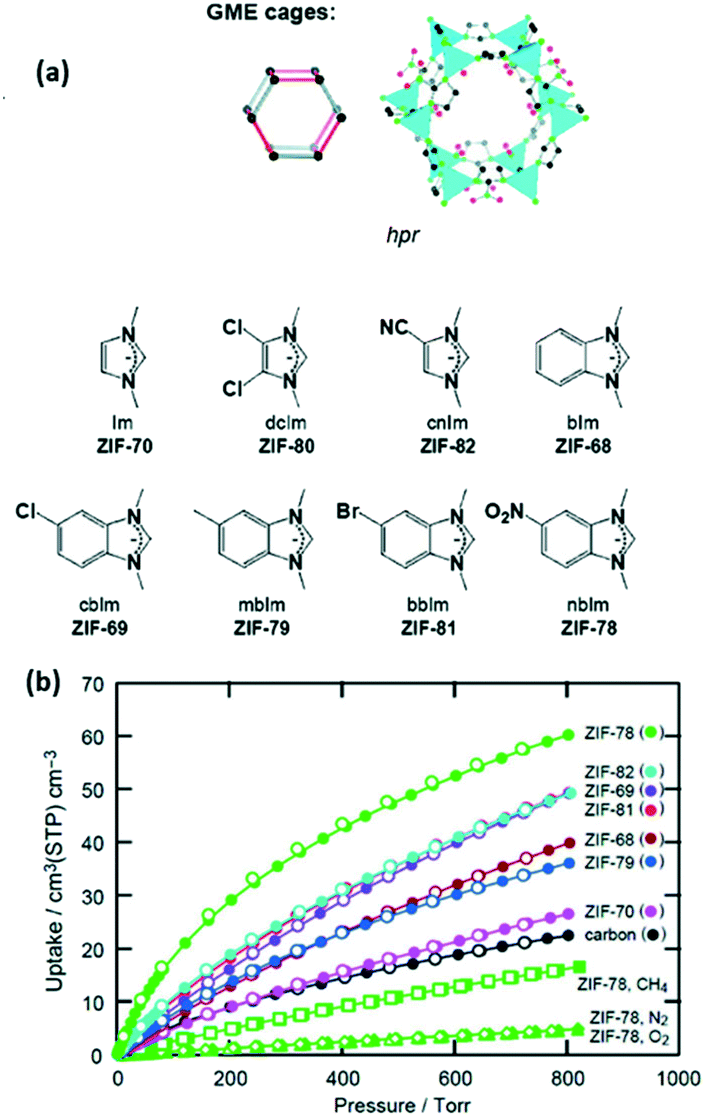
Understanding The Opportunities Of Metal Organic Frameworks Mofs For Co 2 Capture And Gas Phase Co 2 Conversion Processes A Comprehensive Overview Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 D1re00034a
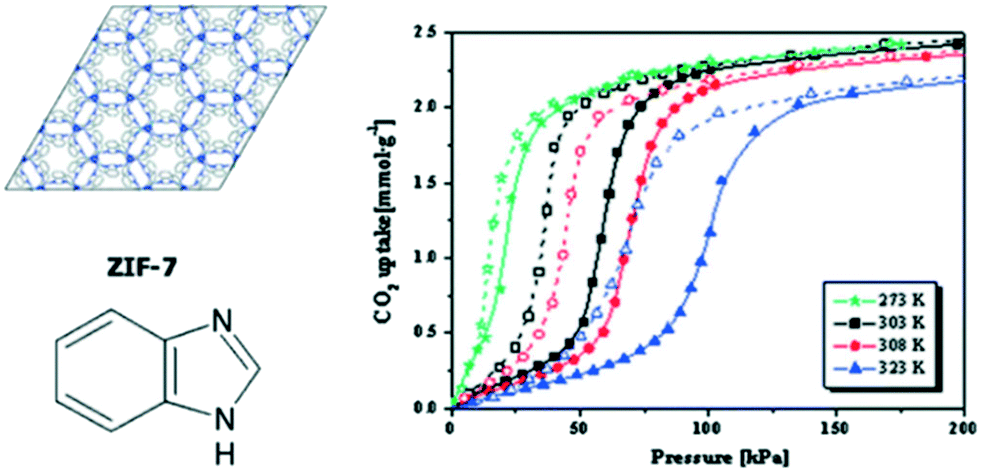
Understanding The Opportunities Of Metal Organic Frameworks Mofs For Co 2 Capture And Gas Phase Co 2 Conversion Processes A Comprehensive Overview Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 D1re00034a
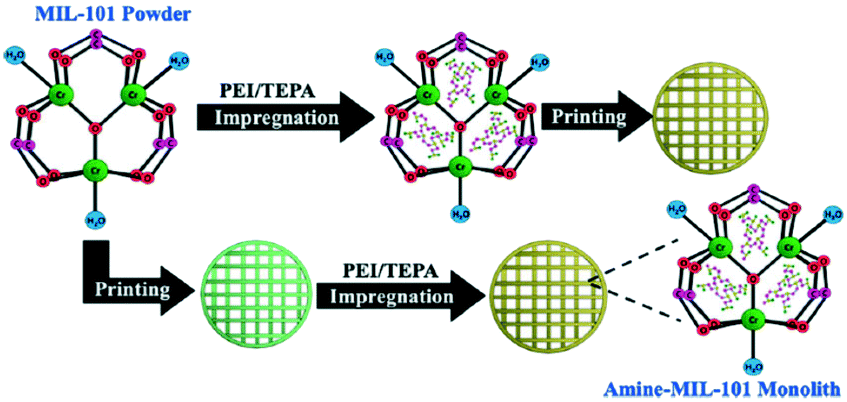
Understanding The Opportunities Of Metal Organic Frameworks Mofs For Co 2 Capture And Gas Phase Co 2 Conversion Processes A Comprehensive Overview Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 D1re00034a

Site Specific Incorporation Of Two Ncaas For Two Color Bioorthogonal Labeling And Crosslinking Of Proteins On Live Mammalian Cells Sciencedirect
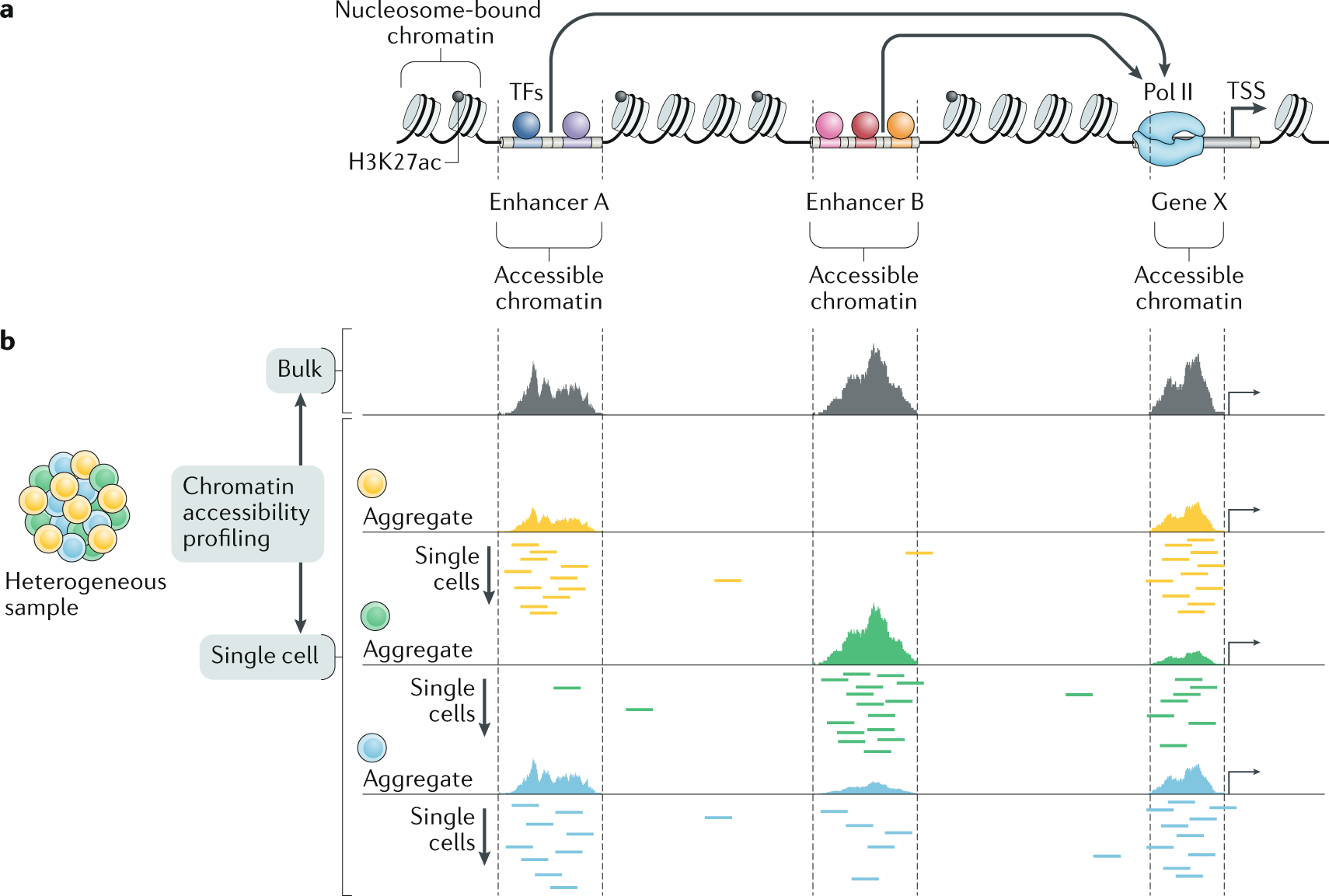
Chromatin Accessibility Profiling Methods Nature Reviews Methods Primers

Shaken Not Stirred Oscillator Drives Electron Spin Technology Org Technology Nanotechnology Electrons
Https Pubs Rsc Org En Content Articlepdf 2018 Gc C8gc00482j

Practices Of Science Scientific Error Manoa Hawaii Edu Exploringourfluidearth

Pre Analytical Errors Their Impact And How To Minimize Them Medical Laboratory Observer

A Guide To Preparation Protocols In Palynology
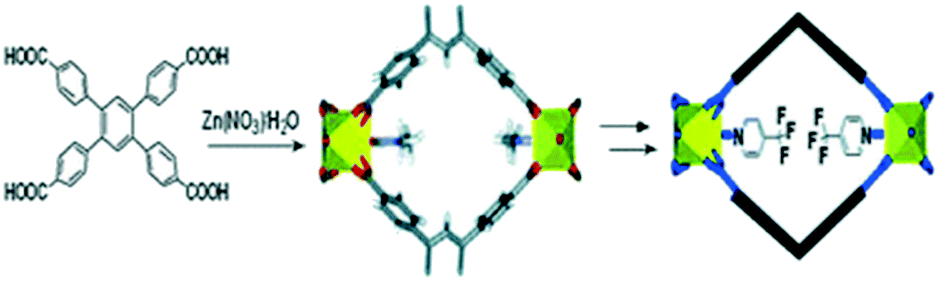
Understanding The Opportunities Of Metal Organic Frameworks Mofs For Co 2 Capture And Gas Phase Co 2 Conversion Processes A Comprehensive Overview Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 D1re00034a
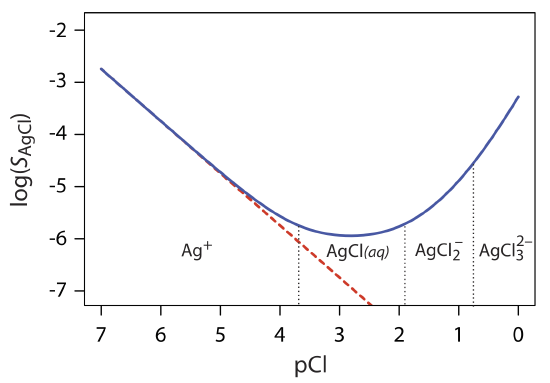
8 2 Precipitation Gravimetry Chemistry Libretexts

Interferences In Clinical Chemistry Analysis
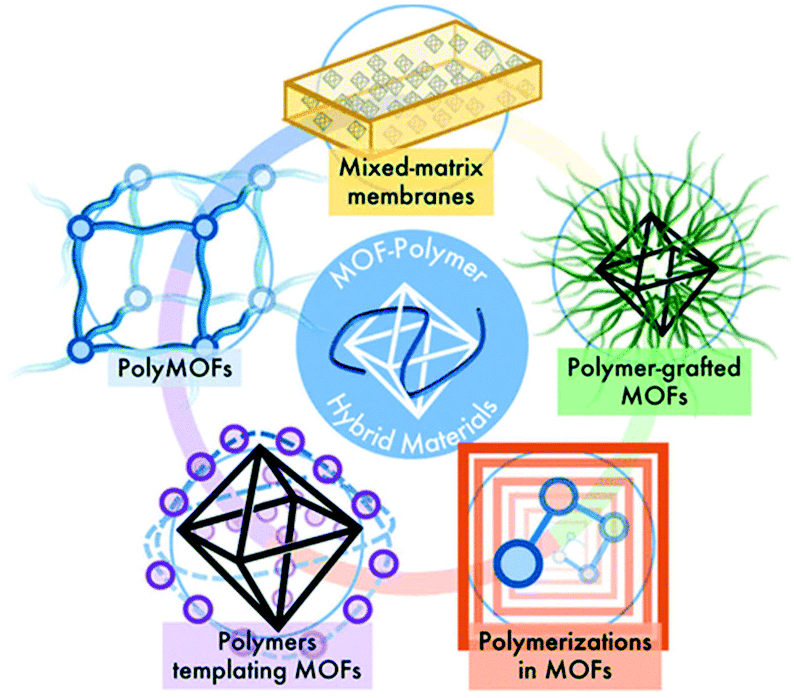
Understanding The Opportunities Of Metal Organic Frameworks Mofs For Co 2 Capture And Gas Phase Co 2 Conversion Processes A Comprehensive Overview Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 D1re00034a

Quantum Chemical Study Of The Fencn Conversion Reaction Mechanism In Lithium And Sodium Ion Batteries Chen 2020 Angewandte Chemie Wiley Online Library
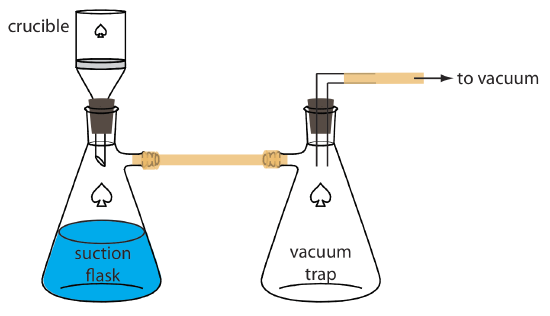
8 2 Precipitation Gravimetry Chemistry Libretexts
Posting Komentar untuk "Unavoidable Errors In Chemistry Labs"