What Does Spontaneous Mean In Chemistry
Chemistry glossary Definition of the spontaneous process and examplesIn a system whether in chemistry biology or physics there are spontaneous and non-spontaneous processes. The entropy of any system is the amount of randomness in it.
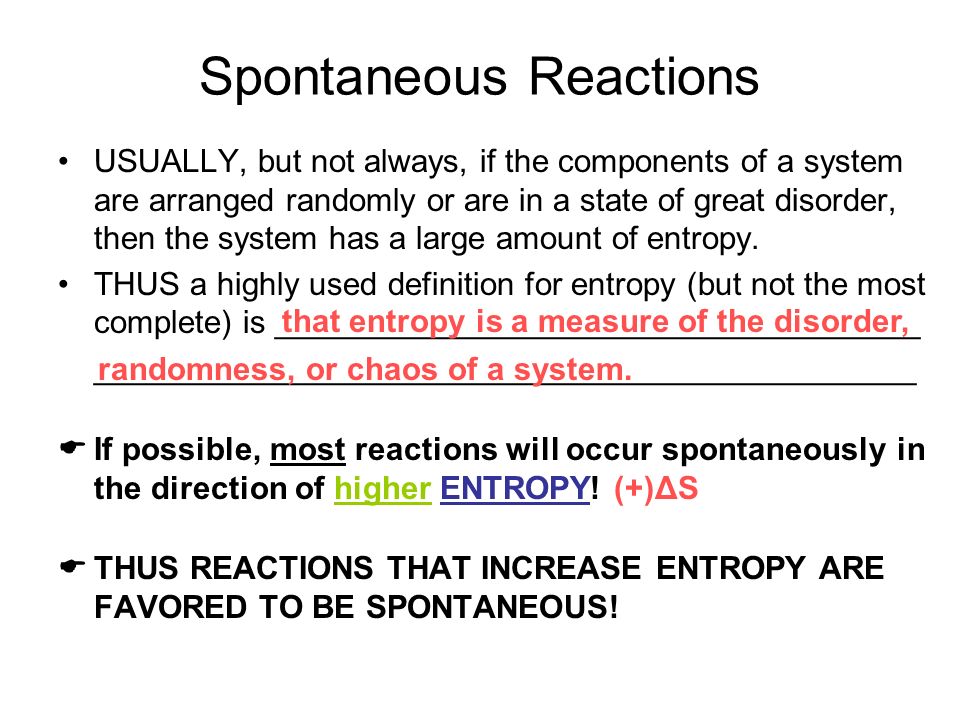
The entropy of any system is defined as the degree of randomness in it.

What does spontaneous mean in chemistry. A roaring bonfire see figure below is an example of a spontaneous reaction. A reaction that is spontaneous will proceed without outside intervention. In order for a reaction to be nonspontaneous it must be endothermic accompanied by a decrease in entropy or both.
Correspondingly what does spontaneous mean in chemistry. The two driving forces of a chemical reaction are enthalpy and entropy. It only takes a minute to sign up.
The reaction is spontaneous. What does non spontaneous mean. Definition of a Spontaneous Process A spontaneous process is one that occurs on its own without any energy input from the outside.
Active 1 year 2 months ago. A spontaneous process is an irreversible process. Enthalpy is a thermodynamic property of a system that is the sum of the internal energy added to the product of the pressure and the volume of the system.
A nonspontaneous reaction is a reaction that does not favor the formation of products at the given set of conditions. Chemistry Stack Exchange is a question and answer site for scientists academics teachers and students in the field of chemistry. The total energy from the reactants reached the activation energy that allow the reaction proceed.
If E oredox reaction is negative E oredox reaction 0 the reaction will not proceed in the forward direction non-spontaneous. If E oredox reaction is positive the reaction will proceed in the forward direction spontaneous. Definition of spontaneous process A spontaneous process is one that will occur without energy from the environment.
The rate of a reaction is independent of its spontaneity and instead depends on the chemical kinetics of. A spontaneous process is an irreversible process and it could only be reversed by some external agents. A reaction that is spontaneous means that it can produce products without the supply of energy.
Spontaneous reactions refer to the chemical reactions that occur without being driven by an outside force. For example the decay of diamonds into graphite is a spontaneous process that occurs very slowly taking millions of years. Spontaneous redox reactions can be used to produce electricity see galvanic cells voltaic cells.
The key word there is. A spontaneous reaction is a chemical reaction that proceeds without any supply of energy. Spontaneity does not imply that the reaction proceeds with great speed.
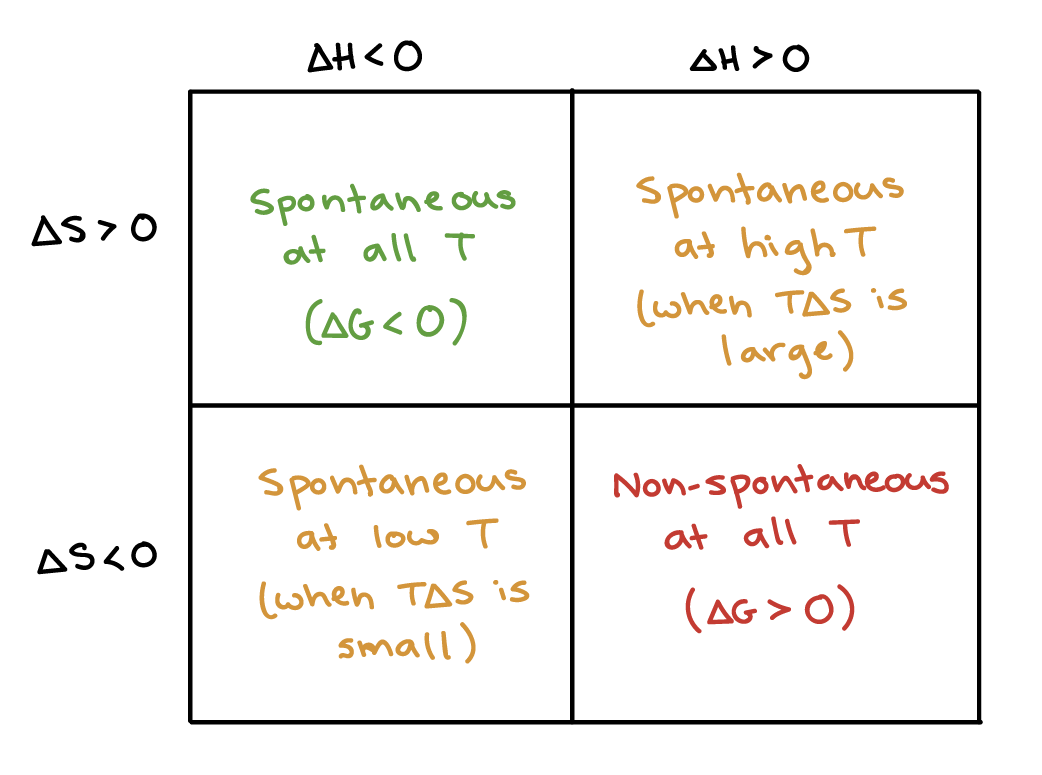
The reaction is non spontaneous. The reaction is spontaneous if S. From this we can define a spontaneous reaction as a reaction that occurs in a given set of conditions without intervention.
What does a positive value of G mean for a reaction. In a system be it chemistry biology or physics there are spontaneous processes and nonspontaneous processes. Spontaneous reactions are accompanied by an increase in overall entropy or disorder.
What does spontaneous mean in term of forward and reverse reactions. How to Predict the Spontaneity of a Reaction. Our atmosphere is composed primarily of a mixture of nitrogen and oxygen gases.
The reaction is spontaneous if S0. A spontaneous reaction is a reaction that occurs in a given set of conditions without intervention. A spontaneous reaction is one that favors the creation of products which is a result of the products being at a lower energy state and having greater entropy than the reactants.
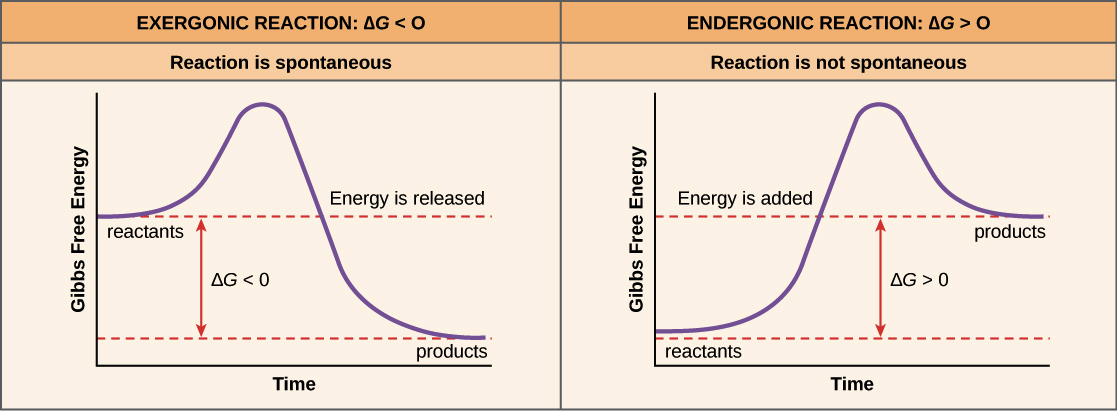
The Gibbs free energy of the products is lower than that of the reactants which implies stable products. A reaction that occurs spontaneously at room temperature has a positive total entropy value and a low activation energy meaning that it is thermodynamically feasible as the products are thermodynamically stable relative to the reactants and kinetically feasible as the products are kinetically stable relative to the reactants thus occurs spontaneously and goes to completion at room. It is a process that will happen on its own.
Once the reaction is started it will continue to completion without additional energy inputs. An example of this is the decay of a diamond into graphite occuring readily. A fire is exothermic which means a decrease in the energy of the system as energy is released to the surroundings as heat.
Spontaneous processes often require activation energy but do not require a prolonged input of energy. Therefore the change in the free energy of the reaction is negative 1-8. Spontaneous processes are reactions which proceed without requiring an input of energy because the products are at a lower more stable energy state than the reactants.
However you can actually reverse it by the application of some external agents. Spontaneous processes spontaneity enthalpy entropy. Ask Question Asked 1 year 2 months ago.
A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.

Spontaneous Processes And Chemical Potential Openstax Chemistry 2e 16 1 Youtube
Spontaneity Free Energy And Temperature Introductory Chemistry
13 7 The Gibbs Free Energy Chemistry Libretexts

Difference Between Spontaneous And Nonspontaneous Reactions Compare The Difference Between Similar Terms
Gibbs Free Energy And Spontaneity Article Khan Academy
Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

Spontaneous Reaction Definition And Examples

Spontaneity Definition Prediction Gibbs Energy Videos Solved Example

Gibbs Free Energy And Spontaneity Video Khan Academy
Difference Between Spontaneous And Nonspontaneous Reactions Definition Thermodynamics Examples Similarities And Differences
Spontaneous Reactions In The Context Of A Chemical Reaction Spontaneous Describes A Reaction That Can Proceed Of Its Own Accord Without Outside Or Ppt Download

Is It A Spontaneous Reaction Delta G Tells You Youtube

19 6 Gibbs Energy Change And Equilibrium Chemistry Libretexts

Difference Between Spontaneous And Nonspontaneous Reactions Compare The Difference Between Similar Terms

Spontaneity Definition Prediction Gibbs Energy Videos Solved Example


Posting Komentar untuk "What Does Spontaneous Mean In Chemistry"