Acid Base Titration Lab Sources Of Error
Alkanes and Alkenes Organic Chemistry. There are plethora of sources of errors to occur in due course of titration.

Difference Between Gene Mutation And Chromosomal Mutation Definition Features Types Similarities And Differences Teaching Biology Biology Science Biology
Several factors can cause errors in titration findings including misreading volumes mistaken concentration values or faulty technique.

Acid base titration lab sources of error. Care must be taken as the solution of the known concentration is introduced into a specific volume of the unknown through laboratory glassware such as a burette or pipette. Acid Base Titration Sources of Error Improvements Sciencing Chemists use acid-base reactions in conjunction with an indicator a compound that changes color when in acidic or basic conditions to analyze the amount of acid or base in a substance. A Excess water in the conical flask will have no influence whatsoever in first order approximation.
Rinse the burette with H 2O. Failure to titrate beyond the equivalence point making determining the point at which the two solutes had completely reacted impossible. Drawing Cyclohexane Rings Organic Chemistry.
It can be either of end point error misreading volumes concentrations faulty use of equipment contaminated glass ware etc. ACID BASE TITRATION OBJECTIVES 1. Discover what titration is and how to calculate the concentration of an acid or base that has been titrated to equivalence.
The Words of Chemistry. Discard in waste container. It can be either of end point error misreading volumes concentrations faulty use of equipment contaminated glass ware etc.
To learn to calculate molarity based on titrations INTRODUCTION Molarity M or molar concentration is a common unit for expressing the concentration of solutions. Using wrong amount of indicator - as discussed in the acid-base titration indicators section in the case of single color indicators amount added can shift end point. The first section is a.
To demonstrate the basic laboratory technique of titration 2. Possible sources of error include. Introduction to Organic Chemistry.
In this experiment only the Calcium Carbonate was considered in the chemical formula however there may have been other ingredients within the tablet that could influence the concentration of substances of the reaction. The titration of a strong acid with a weak base will result in an endpoint in the acidic range. It can be either of end point error misreading volumes concentrations faulty use of equipment contaminated glass ware etc.
Learn the meaning of titrant standard solution and equivalence point. The standard base was made using an analytical. Another source of error came not precisely adding exactly 02 mL titrant each interval which slightly flawed the titration curve.
In this regard what are systematic errors in titration. There are plethora of sources of errors to occur in due course of titration. Failure to properly measure or standardize the concentration of the NaOH solution.
Click to see full answer Also question is what are some experimental errors in titration. The final source of error could have been due to the composition of the antacid tablet itself. Balance used in a titration experiment are technically sources of systematic error these errors are easily avoided.
There are plethora of sources of errors to occur in due course of titration. The first source of error came from possibly misreading the molarity of the stock solution NaOH which would make the calculations slightly less accurate. Rinse with.
The following discussion the errors in a titration experiment are considered. Check valve and seal. Since you are determining the the amount of substance of methanoic acid with the titration the volume is irrelevant.
CHEMISTRY 11 Acid-Base Titration FULL FORMAL LAB Toombs Titration Lab Procedure DAY ONE. Click here to know more about it. It can be either of end point error misreading volumes concentrations faulty use of equipment contaminated glass ware etc.
The volume you use to determine the concentration is from when you take the aliquot with the pipette. Using dirty glass - if glass was not properly cleaned before use it may be contaminated with old reagents which can react with new ones changing their concentration. The titration of a strong base with a weak acid shifts the endpoint towards the alkaline range.
Some of errors are. The calibration of the balances is periodically checked using a registered. Use a beaker filled with H 2O to perform these rinses and then dump the water rinsings into the sink 2.
Several factors can cause errors in titration findings including misreading volumes mistaken concentration values or faulty technique. DO NOT put the buret under the water tap. Misjudging the color of the indicator near.
There are plethora of sources of errors to occur in due course of titration. This explains why several different indicators are used in acid-base titrations.
Https Www Cerritos Edu Chemistry Includes Docs Chem 111 Lab Exp 2012 B Sp 2007 Pdf

Titration End Point Decor Home Decor Lamp
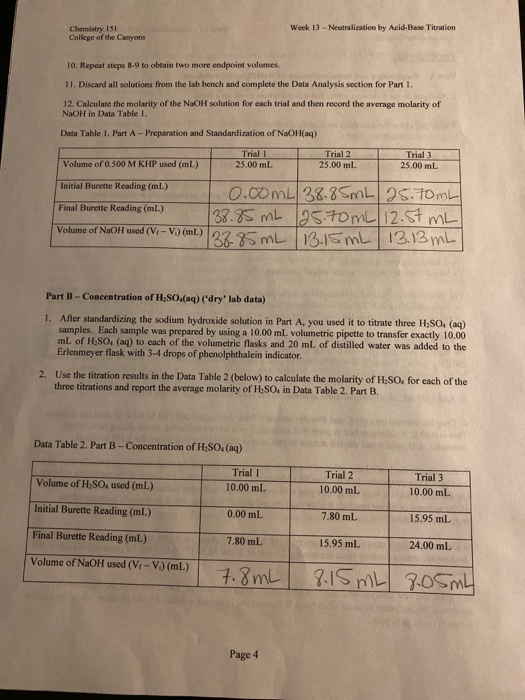
Chemistry 151 College Of The Canyons Week 13 Chegg Com
Lab Report Write Up Tom Green The Channel

Questions For Lab Report While Doing A Titration It Chegg Com

Lab 7 An Introduction To Titrations Posted The Lab Chegg Com

What Are Some Sources Of Errors In Titration Quora

Titrating Sodium Hydroxide With Hydrochloric Acid Experiment Rsc Education

Chemistry Lab Report On Standardization Of Acid And Bases

Error 404 Page Not Found Chemistry Lab Equipment Chemistry Classroom Science Tools

Titration Chemwiki Teaching Chemistry Chemistry Lessons Science Chemistry

Formal Lab Report Of Vinegar Lab Chem C125 Experimental Chemistry Studocu
Post Lab Question To Be Included In The Discussion Chegg Com
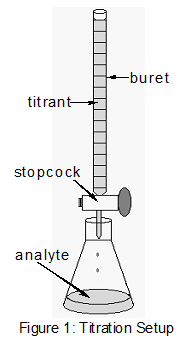

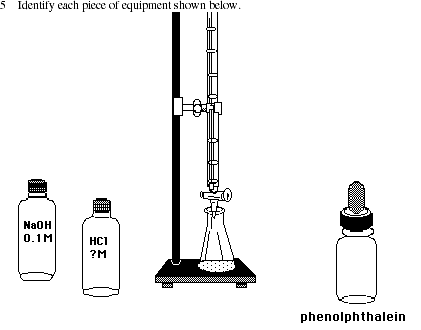


Posting Komentar untuk "Acid Base Titration Lab Sources Of Error"