1st Law Of Thermodynamics For Closed System
The first law is applied first to an adiabatic closed system and then to a non-adiabatic closed system. Internal energy change can be considered as a measure of molecular.

Cycle 2 Week 19 Science First Law Of Thermodynamics Internal Energy Youtube Internal Energy Thermodynamics Physical Science High School
The the volume of the system stays constant which is the case with a.

1st law of thermodynamics for closed system. Q 1-2 W 1-2 ΔU. The change in stored energy for the system is Now the conservation of energy principle or First Law of Thermodynamics for Closed Systemsis written as If the system does not move with a velocity and has no change in elevation the conservation of energy equation reduces to We will find that this is the most commonly used from of the fist law. ΔE ΔKEΔP E ΔU Q W.
The first law of thermodynamics states that the energy of the universe is constant. 1change of internal energy 2 work done by the system. During an interaction between a system and its surroundings the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings.
If we consider only PΔV work above equation becomes. If a closed system undergoes a change of state or a process and during which both work transfer and heat transfer are involved then the net energy transfer will be stored within the system. Here we learn to use a procedure that will help us to systematically solve problems.
A closed system can exchange energy with its surroundings through heat and work transfer. Δ E Δ K E Δ. From first law of thermodynamics it can be said that the internal energy of the system remains same and it is equal to the heat provided to the system minus the work done by the system.
This states that for the frictionless case the supply of pressure-volume work W v and heat Q leads to a change in the internal energy ΔU of the substance. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The laws of closed systems can therefore be used as a basis for the calculation of the pressure-volume work in particular the first law of thermodynamics for a substance in a closed system.
SE MECHANICAL SEM 3 THERMODYNAMICS Module 1 Basic Concepts First Law of Thermodynamics 12g Application of First Law of Thermodynamics to Closed System or Non Flow Process Previous Topic Back to Lesson Next Topic. First law of Thermodynamics for a Closed System Work done for a closed system is the product of pressure applied and the change in volume that occurs due to applied pressure. Hence for a finite non-cyclic process first law of thermodynamics becomes.
For a closed system in equilibrium KE PE and other kinds of stored energy are zero. The change in the internal energy of a system is the sum of the heat transferred and the work done. The First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle.
For a closed system no mass transfer process proceeding between two states. First Law for a Closed System Undergoing a Process. The first law of thermodynamics can be simply stated as follows.
The value of the specific heats for solids and liquids are available in literature Sonntag and Borgnakke 2012 Table A335 52 The first law for steady state open systems Consider an open system with mass flowing in and out of the system. The first law of thermodynamics is the simple the law of energy conservation. We consider the First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle.
W P ΔV Where P is the constant external pressure on the system and ΔV is the change in volume of the system. It says the energy you provide to any system will be used in two ways. Thus energy is transferred between the system and the surroundings in the form of heat and work resulting in a change of internal energy of the system.
If it is a constant volume process then the work done is zero because there is no displacement. In this lesson you learn about the first law of thermodynamics also known as the conservation of energy principal. In this video we solve for the heat transfer of a closed piston-cylinder assembly undergoing a polytropic process using the 1st Law of Thermodynamics.
The heat flow is equal to the change in the internal energy of the system plus the PV work done.

Entropy Balance For Open Systems Thermodynamics Entropy Potential Energy

Imp Snell S Law And Refraction Lenses Reflection And Refraction Refraction Reflection

Chemical Thermodynamics The First Law Thermodynamics Chemistry Education Physics And Mathematics
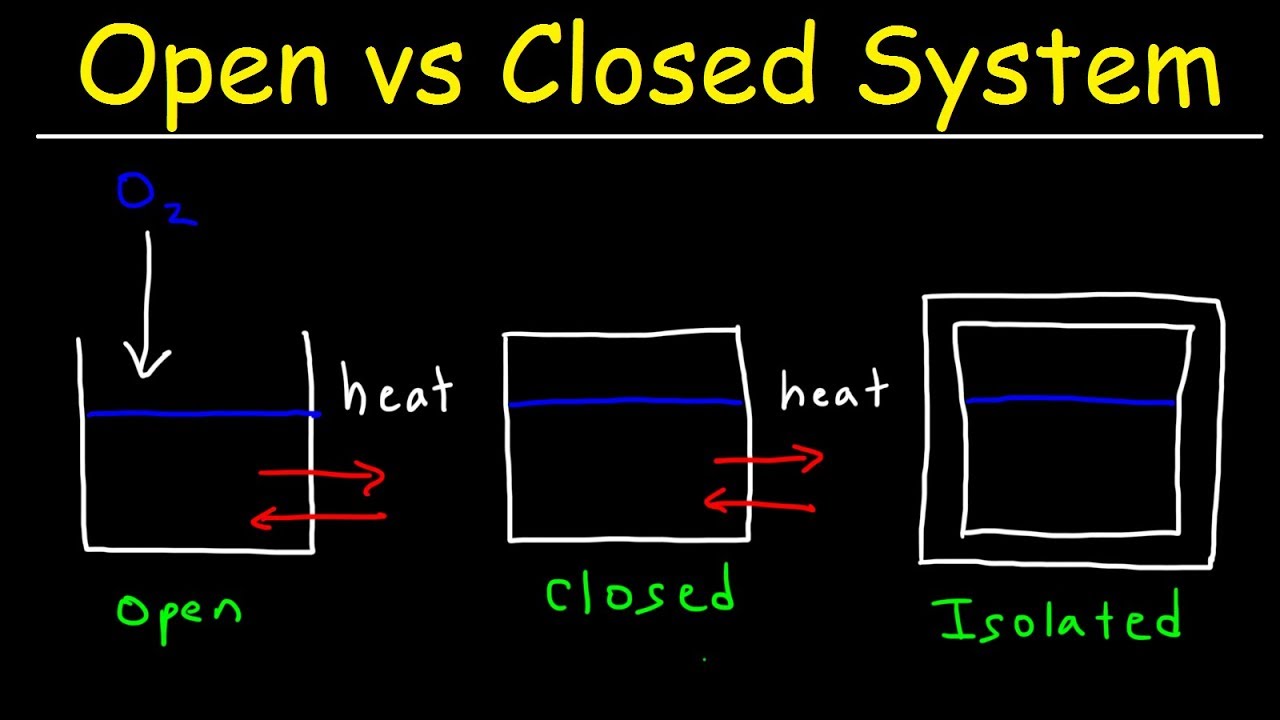
Open System Closed System And Isolated System Thermodynamics Physics Thermodynamics Physics Chemistry Education

First Law Of Thermodynamics Infographic Thermodynamics Ap Physics Chemistry Help

First Law Of Thermodynamics Thermodynamics Highschool Life Science Facts

1st Law Of Thermodynamics System Types Thermodynamics System Heat Transfer

Entropy And Second Law Of Thermodynamics Second Law Of Thermodynamics Thermodynamics Apologia Chemistry

First Law Of Thermodynamics Thermodynamics Energy Transfer Engineering

4 Laws Of Thermodynamics Explained Thermodynamics Science Anchor Charts Physics Formulas

Physics Is Awful Thermodynamics Losing Faith Faith In Humanity

The Laws Of Thermodynamics Chemistry Education Thermodynamics Engineering Science

Mechanical Engineering Youtube Second Law Of Thermodynamics Free Energy Thermodynamics

As A Tattoo Physical Chemistry Chemistry Basics Thermodynamics

First Law Of Thermodynamics Video In 2021 Thermodynamics Internal Energy Law

First Law Of Thermodynamics Internal Energy Heat And Work Youtube Internal Energy Thermodynamics Potential Energy

First Law Of Thermodynamics Video In 2021 Thermodynamics Internal Energy Law
Posting Komentar untuk "1st Law Of Thermodynamics For Closed System"