Protons And Electrons Have A Basic Property Called
Neutrons have no net charge. Atomic Particles Atoms consist of three basic particles.
Atom Atomic Mass And Isotopes Britannica
The nucleus is composed of protons and neutrons not shown.
Protons and electrons have a basic property called. Protons are positively charged particles. Protons have a positive charge. Protons carry a positive electrical charge electrons carry a negative electrical charge and neutrons carry no electrical charge at all.
Electron properties Electrons have three fundamental properties. Charge mass and spin. Each electron has a negative.
Electrons have an electric charge of 1602 176 634 10 19 coulombs which is used as a standard unit of charge for subatomic particles and is also called the elementary charge. It is made up of protons and neutrons. Atoms consist of protons neutrons and electrons.
Protons and neutrons are not called fundamental particles because they are constructed of quarks. Start studying Chapter 2. The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force.
The nucleus contains protons and neutrons. Given below in a table is the comparison between protons neutrons and electrons. All leptons have an electric charge of 1 or 0.
Atoms are made of extremely tiny particles called protons neutrons and electrons. An atom are protons neutrons and electrons. Electrons move around the nucleus in orbits similar to the way planets move around the sun.
The negative charge of the electrons is balanced by the positive. The mass of an electron has been measured and found to be 9109389 10 31 kilograms. Orbiting around the nucleus at a very high speed are electrons.
Rubbing silk cloth on glass. Protons and neutrons are in the centre of the atom making up the nucleus. The outermost regions of the atom are called electron shells and contain the electrons negatively charged.
The amount or magnitude of charge on protons is the same as electrons. The center of an atom is called the Nucleus and consists of Neutrons and. Electrons protons and neutrons are still called elementary particles a term left over from the days when they were all thought to be fundamental.
Electrons surround the nucleus. They are a type of fundamental particles called leptons. Each proton has a single positive charge.
Both protons and electrons have a basic property called charge. If an atom has more or fewer electrons than protons then it has an overall negative or positive charge respectively such atoms are called ions. By definition the electric charge on an electron is 1.
Learn vocabulary terms and more with flashcards games and other study tools. This makes electrons and protons stick together to. Together these are called nucleons.
Those regions are now called energy levels. The other two types are protons and neutrons. Electrons are negatively charged while protons are positively charged.
Electrons have a negative electric charge and protons have a positive electric charge. Unlike protons and neutrons which consist of smaller simpler particles electrons are fundamental particles that do not consist of smaller particles. The protons and neutrons cluster together in the central part of the atom called the nucleus and the electrons orbit the nucleus.
Things that are negatively charged and things that are positively charged pull on attract each other. Electrons and quarks are fundamental. Atoms have a nucleus with electrons moving around it.
Neutrons on the other hand have a mass similar to that of a proton but has not electric charge. Electrons surround the nucleus. Protons neutrons whole nuclei and electrons all possess spin and are often represented as tiny spinning balls.
Imbalance in a substance between the number of protons and electrons Known as static electricity Protons and electrons are not in motion Caused by an external force Example. Electrons have a negative charge. Electric charge is a basic property of electrons protons and other subatomic particles.
The property of water that makes it possible for water exist as a liquid is called. Protons and neutrons are in the center of the atom making up the nucleus. Protons have a positive charge.
In their neutral state atoms have an equal number of electrons and protons. Charge however can be measured and it affects the behavior of particles. Atomic and subatomic particles posses a corresponding property known as spin or spin angular momentum.
The term doesnt mean much anymore. These three particles are compared with their properties and listed below. Electrons have a negative charge.
The nucleus center of the atom contains the protons positively charged and the neutrons no charge. The solid mass of material at the center of the atom is called the nucleus. Protons electrons and neutrons.
If the number of protons and electrons are equal then the atom is electrically neutral. Within the limits of experimental accuracy the electron charge is identical to the charge of. Electrons also spin on their axes in much the same way that.
This property is not like other physical properties of matter. Although inaccurate this isnt a terribly bad way to think about spin as long as you dont take the analogy too far.
What Is Electric Charge And How Electricity Works Howtomechatronics
The Structure Of The Atom Boundless Chemistry
Introduction To Basic Chemistry Protons Neutrons Electrons And
Structure Reactivity Atoms Protons Neutrons Electrons
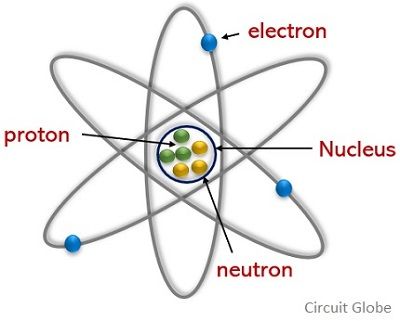
Difference Between Electron And Proton With Comparison Chart Circuit Globe
What Is Electricity Learn Sparkfun Com
Biomolecules And The Chemistry Of Life Protons Neutrons And Electrons Shmoop
What Are The Characteristics Of Electron Proton And Neutron A Plus Topper
Atoms Definition Overview Expii
The Structure Of The Atom Boundless Chemistry
Difference Between Proton Neutron And Electrons
What Is Electricity Learn Sparkfun Com
4 4 The Properties Of Protons Neutrons And Electrons Chemistry Libretexts


Posting Komentar untuk "Protons And Electrons Have A Basic Property Called"