What Two Types Of Particles Exist Within An Atomic Nucleus
The force with which two protons two neutrons or a proton and a neutron attract one another in the nucleus is called nuclear force There are two types of particles in the nucleus of an atom. It consists of two types of subatomic particles packed tightly together.
What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic
The only exception in which it forms an atomic nucleus without any neutrons is the nucleus of ordinary hydrogen - the most abundant nuclide in the universe.
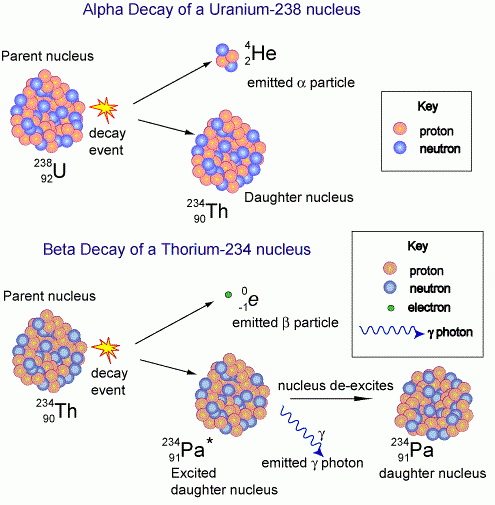
What two types of particles exist within an atomic nucleus. A sub-atomic particle similar in size to a proton and also found nucleus of an atom that has no 0 electric charge when compared to a proton or electron. One Neutron is equal to one Atomic Mass Unit. What can you conclude from this fact.
Atoms consist of three basic particles. Whereas quarks together form nucleons within the atomic nucleus the electrons generally circulate toward. The particles are protons which have a positive electric charge and neutrons which are neutral in electric charge.
The atomic nucleus concentrate almost all the mass of an atom. Atomic nuclei would fly apart. The nucleus center of the atom contains the protons positively charged and the neutrons no charge.
What might happen if the strong force didnt exist. An atom of oxygen has 8 protons. Protons electrons and neutrons.
What two types of particles exist within an atomic nucleus. The number of protons in a nucleus is the same for all the atoms of a particular element. However hydrogen has other isotopes that do contain neutrons.
Inside the nucleus of an atom are typically protons and neutrons sometimes generically called nucleon when someone isnt specifying one or the other species of particle but referring to any random particle in the nucleus. 11 rows Atomic nuclei consist of protons and neutrons. The nucleus plural nuclei is a positively charged region at the center of the atom.
The proton has a positive charge and a large mass 1800 times more than an electron see table 1. The rest of the pack is distributed among the electrons. Protons and neutrons which taken together are called nucleons.
Although both are elementary particles electrons and quarks differ in several respects. However the electrons weigh very little than the neutrons and protons. Like a proton it is the other of the two types of particles that contribute to the mass of an atom.
An atoms nucleus comprises sub-particles called nucleons that can be of two types. The outermost regions of the atom are called electron shells and contain the electrons negatively charged. There are two types of particle in the nucleus of an atom.
Protons are present in atomic nuclei generally attached to neutrons by strong interaction. Each type of nucleus contains a specific. Oxygen has an atomic number of 8.
The proton and the neutron. Nuclei of small atoms are quite stable but the nuclei of big atoms are quite unstable.
Lesson Explainer The Atomic Nucleus Nagwa
2012 In Infographics How Graphic News Saw The World Physics And Mathematics Physics Theoretical Physics
Atoms Nuclei Elements And Isotopes Science And Technology Facilities Council
Atomic Structure Doodle Note Activity For Middle School And Homeschool Science Video Science Doodles Doodle Notes Science Doodle Notes
Reading The Building Blocks Of Matter Geology
2 4 Structure Of The Atom Science 8 Name Date Block Ppt Download
What Two Particles Are Found In The Nucleus Of An Atom Socratic
How Do We Know How Small An Elementary Particle Is
Atom Rutherford S Nuclear Model Britannica
Introduction To The Atom Let S Talk Science




Posting Komentar untuk "What Two Types Of Particles Exist Within An Atomic Nucleus"