What Causes Diffusion And Osmosis
Osmosis will occur whenever the water concentrations are different on either side of a differentially permeable membrane. In simple diffusion this process proceeds without the aid of a transport protein.
An understanding of osmosis and the intestinal absorption of glucose forms the basis for a simple therapy that has saved millions of lives particularly in less-developed countries.
What causes diffusion and osmosis. Water moves into and out of cells by osmosis. The process continues until the concentration of. The spreading out of molecules from an area where they are highly concentrated to an area where they are less concentrated.
The intake of water in plants is an example of osmosis. A water solution that contains nutrients wastes gases salts and other substances surrounds cells. Diffusion does not depend on solute potential pressure potential or water potential.
Requires no energy expenditure and thus also called passive diffusion. What was the color of the starch solution in the beaker after dialysis. The diffusion and osmosis are characterized by the distribution of phenomena molecules of a body in another body that is in contact with the first or separately but through a membrane semiplasmática.
Furthermore osmosis requires a semi-permeable membrane while diffusion does not. What was the color of the iodine solution in the diffusion bag before and after dialysis. Diffusion is a passive process.
This concept can be applied to many things dye in water is a good visual example. Start studying Diffusion Osmosis Scenario Practice. In plants osmosis is partially responsible for the absorption of soil water and for the elevation of the liquid to the leaves of the plant.
Diffusion will continue until the concentration gradient has been eliminated. Diffusion mainly depends on the presence of other particles. Osmosis is the movement of water from a heavily concentrated area of other stuff salts to a less concentrat area.
Learn vocabulary terms and more with flashcards games and other study tools. Diffusion is the random movement of anything away from its origin. Basic Characteristics of Osmosis.
Osmosis can only function in a liquid medium but diffusion can occur in all three mediums solid liquid and gas. In these countries diarrhea caused by cholera and other intestinal pathogens is a major cause of death of young children. What Causes Diffusion and What Happens During the Process The random movement of molecules existing in any state of solid liquid or gas increases the kinetic energy of the system.
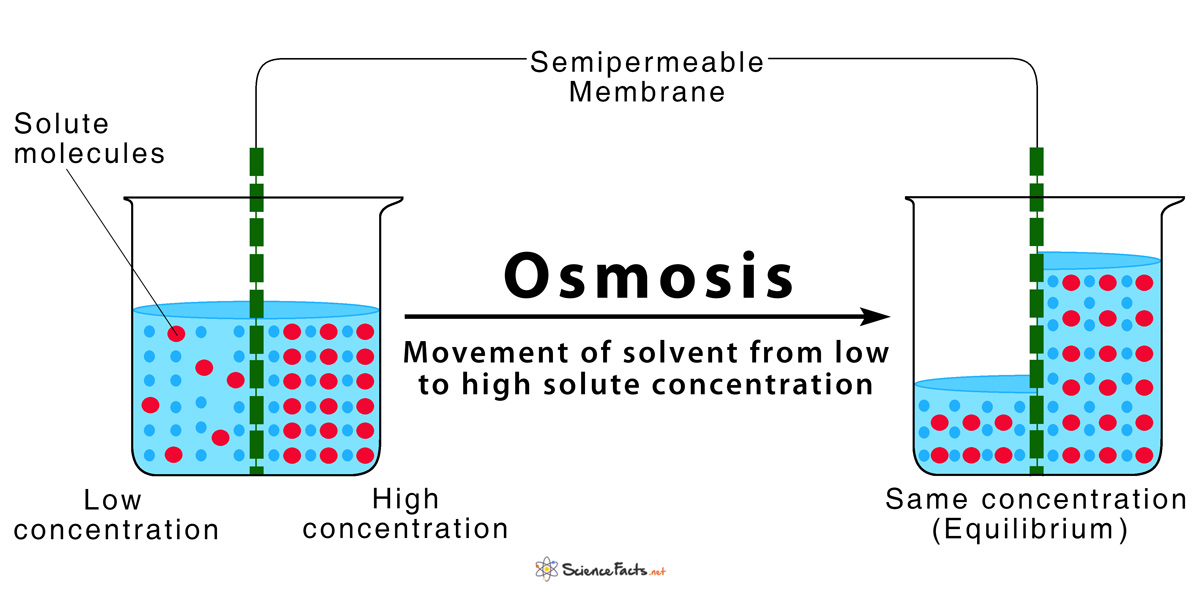
Osmosis is the diffusion of a solvent through a differentially permeable membraneIn biological systems the solvent will usually be water. Biology Diffusion and Osmosis. There is the equalization of.
A simple rule to remember is. Movement of water occurs from a region of high water potential to a region of low water potential. No requirement of semi-permeable membrane for diffusion to occur.
Occurs in liquid medium. While osmosis influences the distribution of nutrients and the release of metabolic waste products in animals. Over time diffusion causes the solution to even up in concentration.
Differences in Function. Osmosis can take place only in a liquid medium. Osmosis mainly depends on the number of solute particles dissolved in the solvent.
Diffusion is observed when a drop of food colouring is added to a glass of water where. Solid liquid or gas diffusion can take place in any of these media. In the cell it is the movement of water molecules.
It is the random motion of the molecules that causes them to move from an area of high concentration to an area with a lower concentration. It will do move until both areas are the same concentration. Semi-permeable membrane is a must for osmosis to take place.
Description of Diffusion and Osmosis. Osmosis - the diffusion of water across a membrane Water will move in the direction where there is a high concentration of solute and hence a lower concentration of water. A slow and spontaneous process.
Osmosis depends on solute potential. Diffusion can occur through a cell membrane and the membrane allows small molecules like water H 2 O oxygen O 2. Osmosis is the diffusion of water molecules across a semipermeable membrane from an area of lower concentration solution ie higher concentration of water to an area of higher concentration solution ie lower concentration of water.
Salt is a solute when it is concentrated inside or outside the cell it will draw the water in its direction. These two possibilities are precisely what opens the division between the two processes. Diffusion is the passive movement of molecules from an area of.
If a cell is in a hypertonic solution the solution has a. Since diffusion equalizes the concentration of the substance on both sides of the region it helps the solution to attain the state of equilibrium or minimum. The iodine solution was reddish brown before dialysis and did not change color during dialysis.
This is the external environment of a cellThe cells outer surface of the plasma membrane is in contact with this external environment while the inner surface is in contact with the cytoplasm. Requires a semipermeable membrane.
Molecular Transport Phenomena Diffusion Osmosis And Related Processes Physics
Molecular Transport Phenomena Diffusion Osmosis And Related Processes Physics
Diffusion And Osmosis Similarities Differences
9 Diffusion And Osmosis Lab Thursday 9 19
Ficks S Law Of Diffusion Osmosis Active Transport Examples In Animal And Plant Cells Isotonic Solution Potato Experiment Osmotic Pressure Igcse O Level Gcse Biology Revision Notes Examinations
What Causes Diffusion Osmosis Filtration 2 If Chegg Com
Osmosis Definition And How Does It Occur With Diagram
Review And Virtual Labs Diffusion And Osmosis Kathy Egbert Library Formative
Difference Between Osmosis And Diffusion In Tabular Form
What S The Difference Between Diffusion And Osmosis
What S The Difference Between Diffusion And Osmosis
What Is Osmosis Definition Types Osmotic Pressure
Osmosis Vs Diffusion How Are They Different From Each Other Science Struck
Diffusion And Osmosis Biology I Laboratory Manual




Posting Komentar untuk "What Causes Diffusion And Osmosis"