Can Oil And Water Be Separated By Evaporation
It is possible to shake up the liquids and get them to mix but they soon separate. Oil and water can be separated by distillation which involves evaporation and condensation of liquids.

Explain The Mixture Separation Techniques Example
Filtration followed by evaporation.

Can oil and water be separated by evaporation. A Identify the mixture from the following. Evaporation It is used to separate those mixtures in which solvent is liquid and solute is soluble solid. Separation of kerosene oil and water is done by a Decantation b Filtration c Condensation d Crystallisation a Decantation.
Wells may start out producing little water but sooner or later all oil wells produce a much larger volume of water than oil. A mixture of oil and water can be separated by filtration. If a mixture of such liquids is allowed to stand for some time they form two separate layers.
There are six ways to separate mixtures including sedimentation decantation filtration evaporation crystallization and distillation. With enough time in a non-turbulent state the differing specific gravities will naturally separate. A mixture of oil and water can be separated by decantation.
The mixture of sugar-salt solution can be separated by evaporation. Cotton fibre is separated. Since oil and water have a different boiling.
Evaporation for Oil Gas Industry T he treatment of produced water can be a major component of the cost of producing oil and gas. Two distinct layers are formed and slowly oil is allowed to flow into another container and is separated from water. The component that forms the top layer can then be separated by decantation.
Separating immiscible liquids is done simply using a separating funnel. The water can be drained off in a separatory funnel. 2 Water can be separated from salt by evaporation.
Oil and water can be separated using a funnel Immiscible means that the liquids dont dissolve in each other oil and water are an example. Answer- False A mixture of oil and water can be separated by Decantation. Let us again consider a mixure of a solid and liquid.
Separation of common salt from water solution d Evaporation. So oil floats on top of water and usually makes a very thin layer covering all the water you have or else it spreads out until the oil layer is just one or two molecules thick and you run out of oil. After that the solution is further filtered and salt will be the residue of.
The elements in the well stream such as oil and water have different gravities. Mixtures that contain only solids must be separated through sublimation extraction magnetic separation or chromatography. It is the method of separation in which liquid solvent or organic solvent evaporates and leaves the solid residue behind.
If we dissolve the mixture in alcohol we will get the salt separated while sugar will be dissolved in alcohol. But they do a tiny bit. A mixture of iron filings and rice flour can be separatedby magnet.
Oil doesnt mix with water and most oils are less dense than water. A mixture of wheat grains and wheat flour can beseparated by sieving. Gravity separation is the most widely used method for oil emulsion separation.
Oil and water dont mix much. The density differences allow water to separate by gravity. Sodium chloride can be separated from rock salt by first adding water to the mixture to dissolve the sodium chloride.
Become a member and. If the water is completely evaporated we will get separated sugar from the mixture. Water can be separated from salt by evaporation.
Mixtures are made up of both solids and liquids. Using any of the oil and water separation methods mentioned above such as absorbance bioremediation centrifugal separation decantation distillation and freezing can definitely help you accomplish your goal of separating any mixture of oil and water quickly and more conveniently. See full answer below.
If kerosene and water are mixed throughly say in a blender they will separate into a kerosene layer on top and a water layer below. As the name suggests evaporation is the process of conversion of water into vapour. For example oil and water from their mixture can be separated by this process.
Oil being lighter than water will float on it. Chalk powder suspension in water can be separated by a Filtration b Evaporation c Condensation d Decantation a Filtration. Oil evaporates very slowly.
The components of a solution of sugar in water can be separated by a filtration b crystallisation c decantation d sedimentation. Rocks are pure substances. The separation then takes place in two stages.
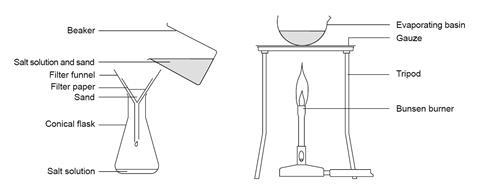
Separating Sand And Salt By Filtering And Evaporation Experiment Rsc Education

Mixtures Solutions Presentation Filtration Evaporation Scientific Skills Evaporation Separating Mixtures

Separation Of Substances Class 6 Science Chapter 5 Evaporation And Condensation Youtube

Separation Techniques In Chemistry Separation Chemistry Tutoring Flyer

Separating Mixtures S Cool The Revision Website

Methods For Separating Mixtures Chemistry For Non Majors
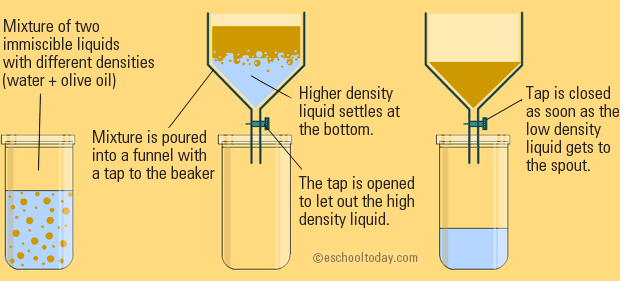
Explain The Mixture Separation Techniques Example

Q16 Give A The Principal Invol Lido

Used Oil Refining Process Oil Water Oil Storage Evaporation

Cambridge Cie Igcse Chemistry Contents Topic 2 Experimental Chemistry Chemistry Evaporation Ap Chemistry
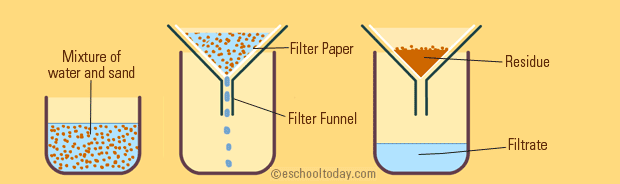
Explain The Mixture Separation Techniques Example

How To Separate A Mixture Of Sugar Water Sugar Crystals Water Projects Mixtures

Major Processes Of Rice Bran Oil Extraction 1 Oil Extraction Process 2 Steam Removal Process 3 Evaporation Rice Bran Oil How To Make Oil Machinery For Sale
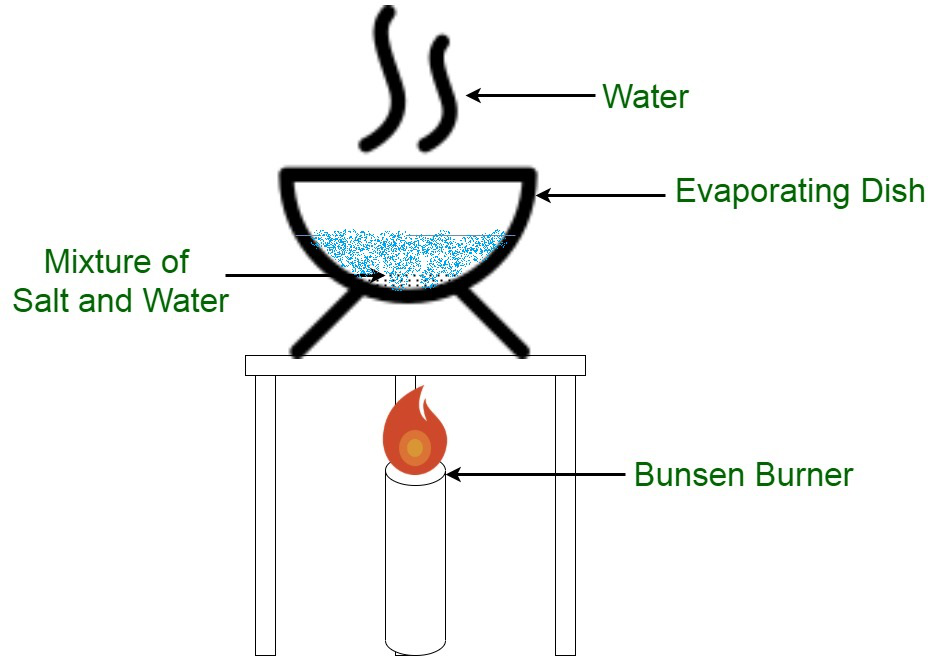
Separation By Evaporation Geeksforgeeks

How To Separate Salt From Sea Water Science Fair Science Experiments Kids Science Activities


Posting Komentar untuk "Can Oil And Water Be Separated By Evaporation"