How To Find Protons And Electrons In An Element
Hydrogen has 1 proton 0 neutron and 1 electron. Uranium for example has the largest naturally occurring nucleus with 92 protons and over 140 neutrons.
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Protons Neutrons Electrons
The number of electrons in a neutral atom is equal to the number of protons.

How to find protons and electrons in an element. A neutral element will have a 0 charge. Helium has 2 protons 2 neutrons and 2 electrons. H is for hydrogen O is for oxygen Na for sodium etc.
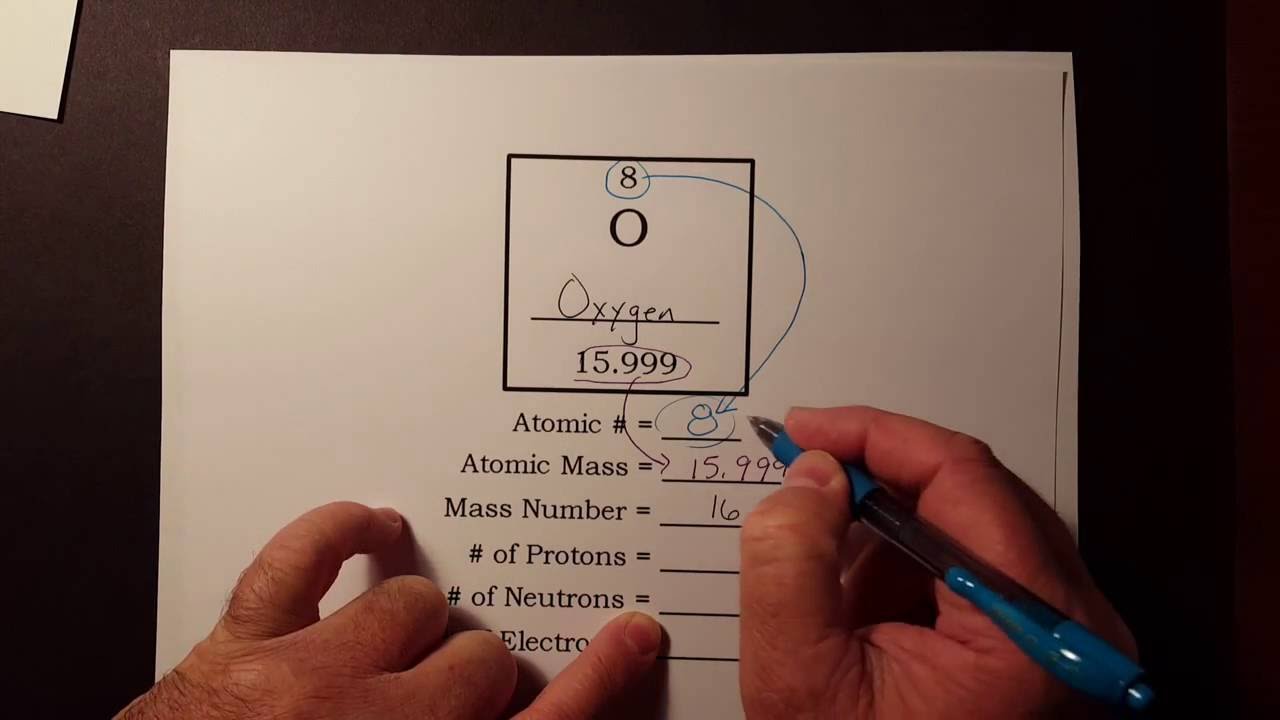
A good Periodic chart should give you enough information to determine the name of the element. The Atomic Number identifies the element and the number of protons. Lithium has 3 protons 4 neutrons and 3 electrons.
112 rows Atomic no. In a balanced atom the number of electrons equals the number of protons. The number of protons is equal to the number of electrons unless theres an ion superscript listed after the element.
The number of protons neutrons and electrons in an atom can be determined from a set of simple rules. You need the atomic number to find the amount of protons andor electrons unless you have the amount of neutrons and the atomic mass in which case you can simply subtract the amount of neutrons from the atomic mass leaving the amount of protons. The easiest way to know how to find number of electrons neutrons and protons for an element is to look at the elements atomic number on the periodic table.
The number of protons in the nucleus of the atom is equal to the atomic number Z. This chemistry video tutorial explains how to calculate the number of protons neutrons and electrons in an atom or in an ion. For example carbon 12 carbon 13 and carbon 14 are three isotopes of Carbon each having 6 electrons.
A positively-charged ion or cation has more protons than electrons. The periodic table is arranged in order of increasing atomic number so the number of protons is the element number. How many protons neutrons and electrons are in each element.
Where A is the mass number of the elements nuclei and Z is the atomic number X stands for the element symbol for example. Carbon has 6 protons 6 neutrons and 6 electrons. What element has most neutrons.
The atomic number is not only equal to the number of protons in a nucleus of an element it is also. In an unbalanced atom the number of electrons equals the number of protons plus the opposite of the ion charge. Periodic Table Basics Learn how to use information from the periodic table to find the number of protons neutrons and electrons of an element.
Protons Neutrons and Electrons of all the Elements. It also explains the differe. No matter how many electrons or neutrons an atom has the element is defined by its number of protons.
Finding Protons Neutrons and Electrons of Isotopes Isotopes are atoms of the same element with the same proton number but different number of neutrons. I tried to make it as simple as possible because I too got confused at one point and I am a student and know how other students minds work. Boron has 5 protons 6 neutrons and 5 electrons.
This number is the atomic number of the element. The proton number is the atomic number of the element while the electron number is the atomic number minus the charge. Beryllium has 4 protons 5 neutrons and 4 electrons.
A neutral atom has the same number of protons and electrons. Boron has 5 protons. How do you determine the number of protons in an element.
In fact its actually possible to have an atom consisting of only a proton ionized hydrogen. Calculate the number of neutrons by subtracting the atomic number from the mass number. That number is equal to the number of protons.
In order to find the electrons take the atomic number and subtract the charge. I am showing how to find proton neutron and electron numbers of atom and ions in this video.

How To Read The Periodic Table High School Chemistry Electrons Electron Configuration

How To Find The Number Of Protons Neutrons And Electrons Neutrons Protons Electrons

How To Find The Number Of Protons Neutrons And Electrons Electrons Protons High School Chemistry

Online Activity Determining How Many Protons Electrons And Neutrons In An Atom Based On The Periodic Science Chemistry Physical Science Homeschool Science

How To Find The Number Of Protons Neutrons And Electrons Neutrons Protons Proton Neutron Electron

New Ap General Chemistry Video Playlist Youtube General Chemistry Protons Neutrons Electrons Protons

What Are The Characteristics Of Electron Proton And Neutron A Plus Topper Https Www Aplustopper Com Characteristics Electron Protons Neutrons Electrons

The Atom Chemistry Is My Jam Atom Protons Chemistry Help

Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic Proton Neutron Electron Protons Neutrons

Atom Ape Man Atomic Mnemonic For Protons Neutrons Electrons Chemistry Worksheets Mnemonics Matter Science

Calculating Parts Of An Atom Practice Worksheet 2 Practices Worksheets Worksheets Chemistry Worksheets

How To Calculate Atomic Mass Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets

How To Find The Number Of Protons Neutrons And Electrons Protons Neutrons Electrons

Iron Atom Element Name Iron Atomic Number 26 Atomic Mass 55 847 Protons 26 Atom Project Science Projects School Projects

See The Electron Configuration Diagrams For Atoms Of The Elements Atom Diagram Electron Configuration Electrons

Protons Neutrons Electrons And Isotopes Youtube Protons Neutrons Electrons

How To Read The Periodic Table Teaching Chemistry Chemistry Classroom Chemistry Education
Posting Komentar untuk "How To Find Protons And Electrons In An Element"