Electrolysis Of Aluminium Oxide Half Equations
The process is based on the use of a powerful electric current to decompose aluminum oxide Al2O3. The balanced half equation is.
2O2- O2 4e- Oxygen is often the anode product from the electrolysis of.

Electrolysis of aluminium oxide half equations. The half equation is. Al3 3e- Al aluminium metal at the -cathode 2O2- - 4e- O2 oxygen gas at the anode Aluminium is more dense than the aluminacryolite solution and so falls to the bottom of the cell where it can be tapped off as pure liquid metal. A half-equation is balanced by adding or taking away a number of electrons equal.
O 2 for the electrolysis of Fe15 experiments are displayed in Fig. It is not balanced. What is the word equation for the electrolysis of Aluminium oxide.
The half equation is. 2 The first step is to purify the bauxite to get pure aluminium oxide Al 2O 3. Aluminum found in the form of bauxite ore it is first converted into pure aluminum oxide by the Bayer Process.
Integral values obtained from the whole duration of electrolysis trials A and B give a mean O 2 of 76 according to Reaction. Extraction of Aluminium Oxide Electrolysis is used to extract Aluminium from Aluminium Oxide Al2O3To do this the Al2O3 must be molten however the melting point of Al2O3 is over 2000 C which is very expensive and inefficient to heat the Al2O3 up that much. The half equation at.
The impurities are iron oxides silicon dioxide and titanium dioxide. Balance the half equation for the formation of aluminium during electrolysis. It shows that oxide ions lose electrons.
1 Bauxite is impure aluminium oxide Al 2O 3. Aluminium oxide electrolysis balanced equation. The half equation is 2O2- O2 4e-.
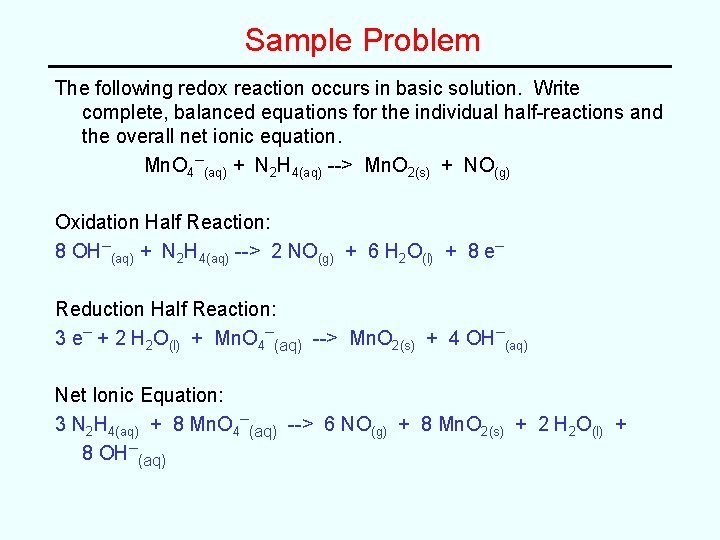
Write an ionic half equation for the reaction at the cathode in electrolysis of molton aluminium oxide HT only Al3 3e- - Al What is the experiment called that allows you to find the concentration of an unknown substance. Al 3 e- Al. 4 Al 3 12 e - 4 Al aluminium metal at the - cathode reduction.
When it is melted the Al 3. Write a balanced half equation for the formation of oxygen from oxide ions in the electrolysis of molten aluminium oxide. Electrolysis of the aluminacryolite solution gives aluminium at the cathode and oxygen at the anode.
Oxygen gas which is given off at the anode reacts with the surface of the aluminium and forms a thick oxide layer. To combat this and make the extraction of aluminum cheaper cryolite is added. This is exactly what happens on a large scale as an intermediate step in the production of aluminum via the H-H process.
The industrial process known as aluminum electrolysis was discovered in 1886 by Paul-Louis Toussaint Héroult and Charles Martin Hall. Electrolysis of the alumina cryolite solution gives aluminium at the cathode and oxygen at the anode. Some electrolysis reactions are carried out in aqueous solution ie.
In the case of aluminum oxide dissolving in water prior to electrolyzing would. Al 3 3e- Al because three negatively charged electrons are. 6 O 2- - 12 e - 3 O 2 oxygen gas at the anode oxidation.
The aluminium metal is treated with sodium hydroxide solution which removes the thin oxide layer on the surface. Explain with the help of a half equation how oxide ions are oxidised during the electrolysis of aluminium oxide. Bauxite is the major aluminium ore.
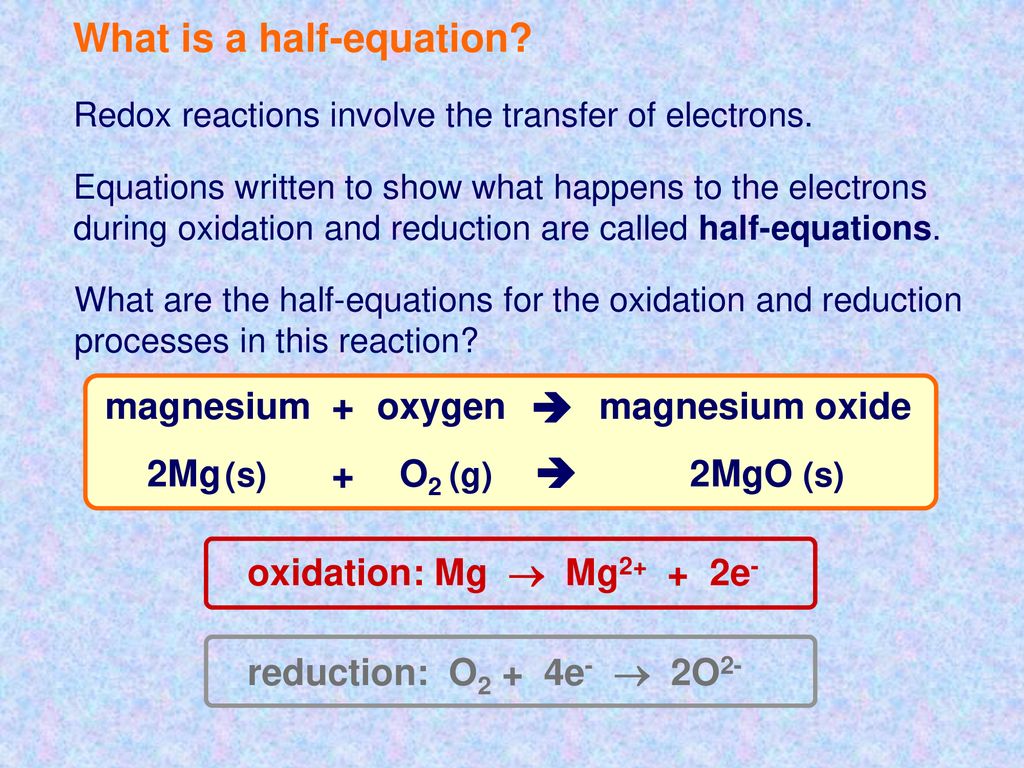
Aluminium oxide Al2O3 is an ionic compound. Look at the equation you have in your question. 2O2- O2 4e-.
Two aluminum hydroxides when heated sufficiently lose three water molecules and form alumina ie A l X 2 O X 3. Electrons are shown as e-. The overall balanced equation for the reaction is.
In these electrolysis experiments O 2 is decreasing with time to values as low as nearly 60. The clean aluminium metal is then used as the anode for the electrolysis of dilute sulfuric acid. Explain with the help of a half equation how aluminium ions are.
3 Molten aluminium oxide is electrolysed in a solution of cryolite Na 3AlF 6 to give out the required. A half-equation shows you what happens at one of the electrodes during electrolysis. 2O 2- O 2 4e -.
The discrepancy can be partially explained by. Explain with the help of a half equation how oxide ions are oxidised during the electrolysis of aluminium oxide. It shows that oxide ions lose electrons and oxidation is loss of electrons.
It shows that oxide ions lose electrons and.

Mountain View Chemical Reactions Chemistry Lessons Chemistry Classroom Chemistry Basics
Oxidation Reduction Reactions Ppt Video Online Download

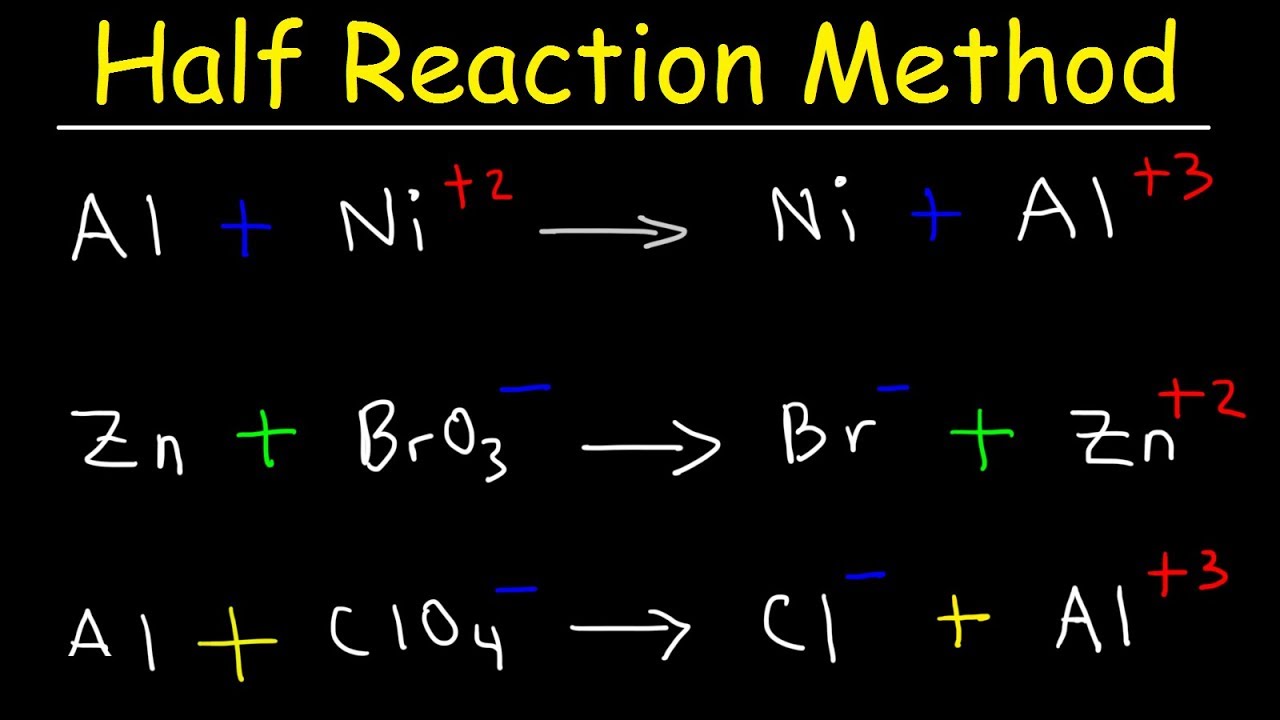
How To Write Ionic Half Equations In Electrolysis Questions Gcse Chemistry Youtube

Electrochemistry Short Notes What Are The Types Of Electrochemical Cells Electrochemical Cell Electrochemistry Redox Reactions
Half Reaction Method Balancing Redox Reactions In Basic Acidic Solution Chemistry Youtube

Extraction Of Aluminium Electrolysis Ionic Equations Anode Replacement

Oxidation Reduction Redox Reaction 2 Redox Reactions Reactions Oxidation
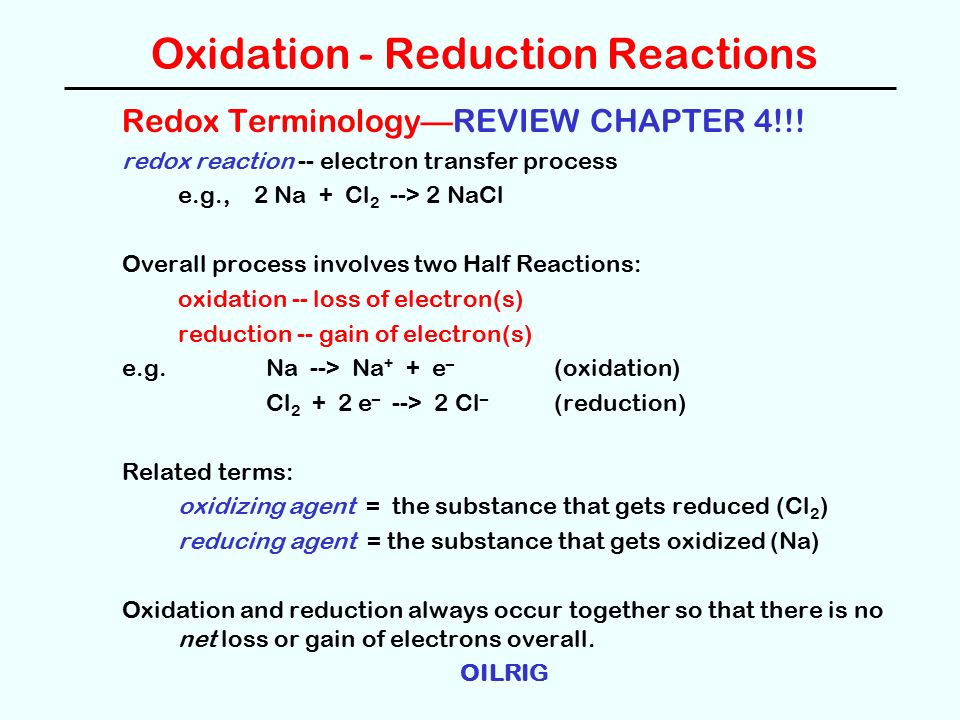
Oxidation Reduction Reactions Redox Terminologyreview Chapter 4 Redox
.jpg)
Half Equations Quiz Questions Footprints Science Gcse Science Animations And Quizzes

Gcse Science Revision Chemistry Electrolysis Of Aluminium Oxide Youtube

Extracting Metals By Electrolysis 1 Of 22 Boardworks

Extraction Of Aluminium Aluminum Recycling Sodium By Electrolysis Raw Materials Bauxite Rock Salt Electrode Equations Description Of Processs Revision Gcse Igcse O Level Ks4 Science Chemistry Revision Notes Revising
Oxidation Reduction Reactions Redox Terminologyreview Chapter 4 Redox


Posting Komentar untuk "Electrolysis Of Aluminium Oxide Half Equations"