Temperature And Kinetic Energy Have A Relationship
Think about the air in a house. 135Phase Changes Interpret a phase diagram.
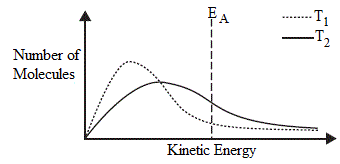
Kinetic Energy Distribution Chemistry Tutorial
Temperature is in fact a measure of the kinetic energy of molecules.
Temperature and kinetic energy have a relationship. Temperature is proportional to heat and is the measure of the average kinetic energy of the molecules in a system. First the average E_ rm k is the average since not all the particles will have identical energies. Define thermal energy.
Esys 3 2 RT. Temperature is a measurement of the average kinetic energy of the molecules in an object or a system. The internal energy of an ideal gas is therefore directly proportional to the temperature of the gas.
Temperature is essentially the speed to which molecules and atoms in a gas are. Heat energy is the energy transferred from. Instead the fundamental definition of temperature is.
It is incorrect to claim something like temperature is a measure of the average kinetic energy in a system which you see very often in popular science and middle-school level textbooks. What is the relationship between kinetic energy and speed. Because the mass of these particles is constant the particles must move faster as the gas becomes warmer.
The absolute temperature of something in Kelvin K is a measure of the average kinetic energy of its particles whether these are atoms molecules or ions. As one increases so does the other. What is the relation between heat temperature and kinetic energy.
The hotter something is the more kinetic energy it has. It may be warmer in one room and cooler in another. Temperature is the average kinetic energy of the particles of matter.
The more mass the more thermal energy there is. Relationship Between Temperature and Kinetic Energy Kinetic energy is the energy possessed by a body due to its motion. Heat temperature and kinetic energy are linked to each other.
We see a range of kinetic energy in molecules because all molecules dont move at the same speed. What is the relationship between temperature and kinetic energy direct or indirect. Kinetic energy is the energy that an object has.
Finally in this model more marbles is a model for more mass. So in this model average kinetic energy represents temperature. Describe the distribution of speeds of molecules in a gas.
In simplest terms when we heat a substance its temperature rises and causes an increase in the kinetic energy of its constituent molecules. What type of relationship do temperature and kinetic energy have group of answer choices. Temperature and kinetic energy have a proportional relationship.
What is the relationship between temperature and kinetic energy quizlet. Note however that despite this close relationship energy and temperature are fundamentally different. Temperature is the average kinetic energy of the particles of matter.
This is a important idea temperature as a characteristic of a system not its individual components. State Daltons law. What is the relationship between temperature and kinetic energy.
Temperature is the average kinetic energy of all the particles. But the total kinetic energy of the molecules of a gas is a measure of. The hotter something is the more kinetic energy it has.
There are two key ideas here. The average kinetic energy in a hot object is higher than the average kinetic energy in a cold object. What is the relationship between temperature and kinetic energy answers Kinetic energy is directly proportional to the temperature applied.
The temperature of a gas is directly proportional to the average kinetic energy of the particles of the gas. Temperature is the average kinetic energy of the particles of matter. When a substance absorbs heat the particles move faster so the average kinetic energy and therefore the temperature increases.
Describe the relationship between the temperature of a gas and the kinetic energy of atoms and molecules. If the degree of motion of the molecules inside an object doubles the temperature will also double. As we see from the equation there is a direct relationship direct proportionality between the kinetic energy and the temperature.
The hotter something is the more kinetic energy it has. Temperature is directly proportional to the average kinetic energy of the molecules in a substance. The key to the kinetic theory of gases is the notion that the average kinetic energy E_ rm k for the particles in a gas sample is proportional to the temperature.
Temperatureis a measure of the average kinetic energy of particles. Populations of molecules have a temperature related to their average velocity but the concept of temperature is not relevant to individual molecules they have kinetic energy but not a temperature. Temperature and kinetic energy have a direct relationship.
Temperature is used as a measure for heat in an object by measuring the amount of kinetic energy in the molecules that make up the object. Some will be lower than average. The average kinetic energy of the particles in a gas is proportional to the temperature of the gas.
The kinetic molecular theory assumes that the temperature of a gas is directly proportional to the average kinetic energy of its particles as shown in the figure below. Calculate the kinetic energy of a gas molecule given its temperature.

The Average Kinetic Energy Per Molecule Equation For An Ideal Gas Ib Physics Youtube
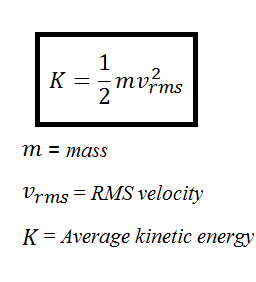
Average Kinetic Energy Temperature Of A System Video Lesson Transcript Study Com

How To Calculate The Average Translational Kinetic Energy Of Molecules Using Boltzmann S Constant Youtube
6 9 Kinetics Chemistry Libretexts
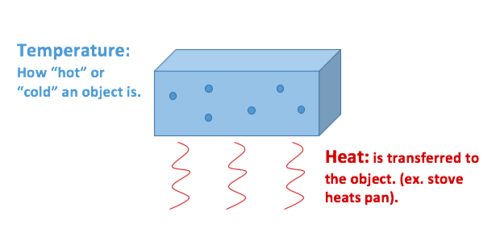
Heat Vs Temperature Energy Education

Average Kinetic Energy Temperature Of A System Video Lesson Transcript Study Com

Is The Average Kinetic Energy Of Evaporating Water Molecules At Room Temperature Equivalent To The Average Kinetic Energy Of Boiling Water Chemistry Stack Exchange
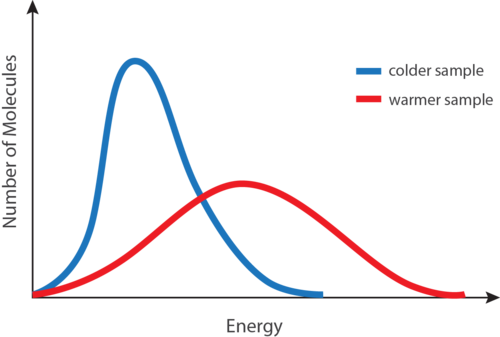
Average Kinetic Energy Chemistry For Non Majors

Energy Enthalpy And The First Law Of Thermodynamics

Average Kinetic Energy Temperature Of A System Video Lesson Transcript Study Com
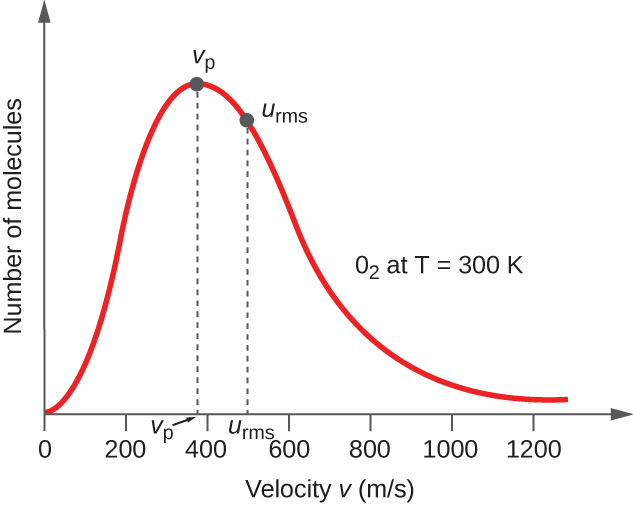
9 5 The Kinetic Molecular Theory Chemistry
Kinetic Molecular Theory Of Gases Introductory Chemistry
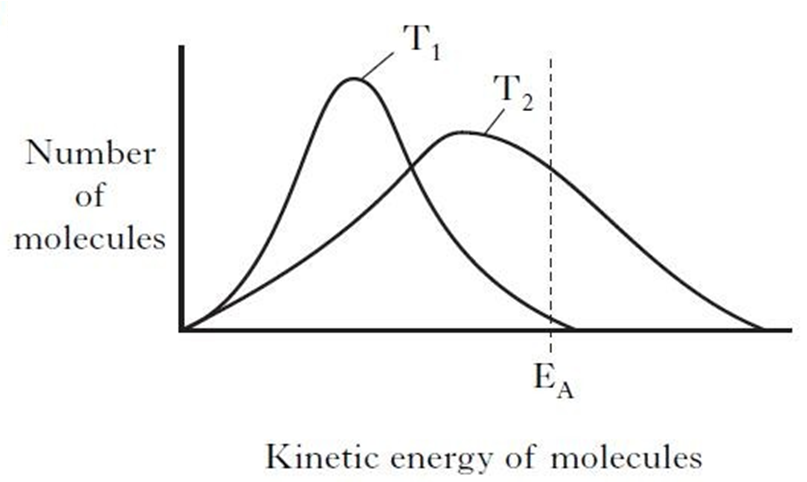
Kinetic Energy Distributions Temperature Higher Chemistry Unit 1

Kinetic Molecular Theory Boundless Chemistry

Posting Komentar untuk "Temperature And Kinetic Energy Have A Relationship"