How Are Temperature And Volume Related
This video will look at Charles Law which describes the relationship between temperature and volume. The volume of a given amount of gas held at constant temperature is inversely proportional to the pressure under which it is measured.
Ideal Gas Equation And Absolute Temperature Boyle S Law Derivation
The relationships among the volume of a gas and its pressure temperature and amount are summarized in Figure 63.

How are temperature and volume related. The Kelvin scale must be used because zero on the Kelvin scale corresponds to a complete stop of molecular motion. Temperature pressure and volume are related for a fixed amount of gas. Charles Law states that the temperature and volume of a gas are directly related.
Measuring Volume For a given mass and volume how much physical space a material takes up of an object or substance the density remains constant at a given temperature. Charless Law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. Volume increases with increasing temperature or amount but decreases with increasing pressure.
Therefore as the temperature of. The volume of a gas is directly proportional to its absolute temperature. The absolute temperature is temperature measured with the Kelvin scale.
Adding the variable of temperature causes the volume and pressure to increase or decrease as the temperature increases or decreases. What is the definition of Moles of Gas and Volume with regards to Avogadros Law. The temperature and volume of one mole an ideal gas during its expansion is related as T T 0αV 2 where T 0 and α positive constants.
Pressure Volume Temperature. P V T n R. This is Charles Law.
The physical behavior of an ideal gas can be expressed in terms of the pressure volume temperature and number of moles of gas present. The Empirically Determined Relationships among Pressure Volume Temperature and Amount of a Gas. The Ideal Gas Law is a single equation which relates the pressure volume temperature and number of moles of an ideal gasIf we substitute in the variable R for the constant the equation becomes.
How is the temperature and the volume of gas related. This is Charles Law. However since volume deviates with changes in temperature and pressure density can also change with temperature and pressure.
R is the universal gas constant. The Ideal Gas Law. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure Boyles law Charles and Gay-Lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure Charless law and Avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present Avogadros law.
The volume is directly to the absolute temperature. The volume of a gas is directly proportional to its absolute temperature. More specifically for a fixed mass of gas at a constant pressure the volume V is directly proportional to the absolute temperature T.
The formula to describe that is a bit more complex including n. If the temperature is in kelvin volume and temperature are directly proportional. The volume and temperature are linearly related for 1 mole of methane gas at a constant pressure of 1 atm.
More specifically for a fixed mass of gas at a constant pressure the volume V is directly proportional to the absolute temperature T.
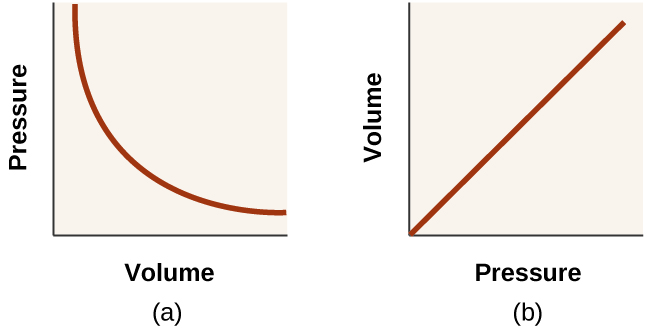
9 2 Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry
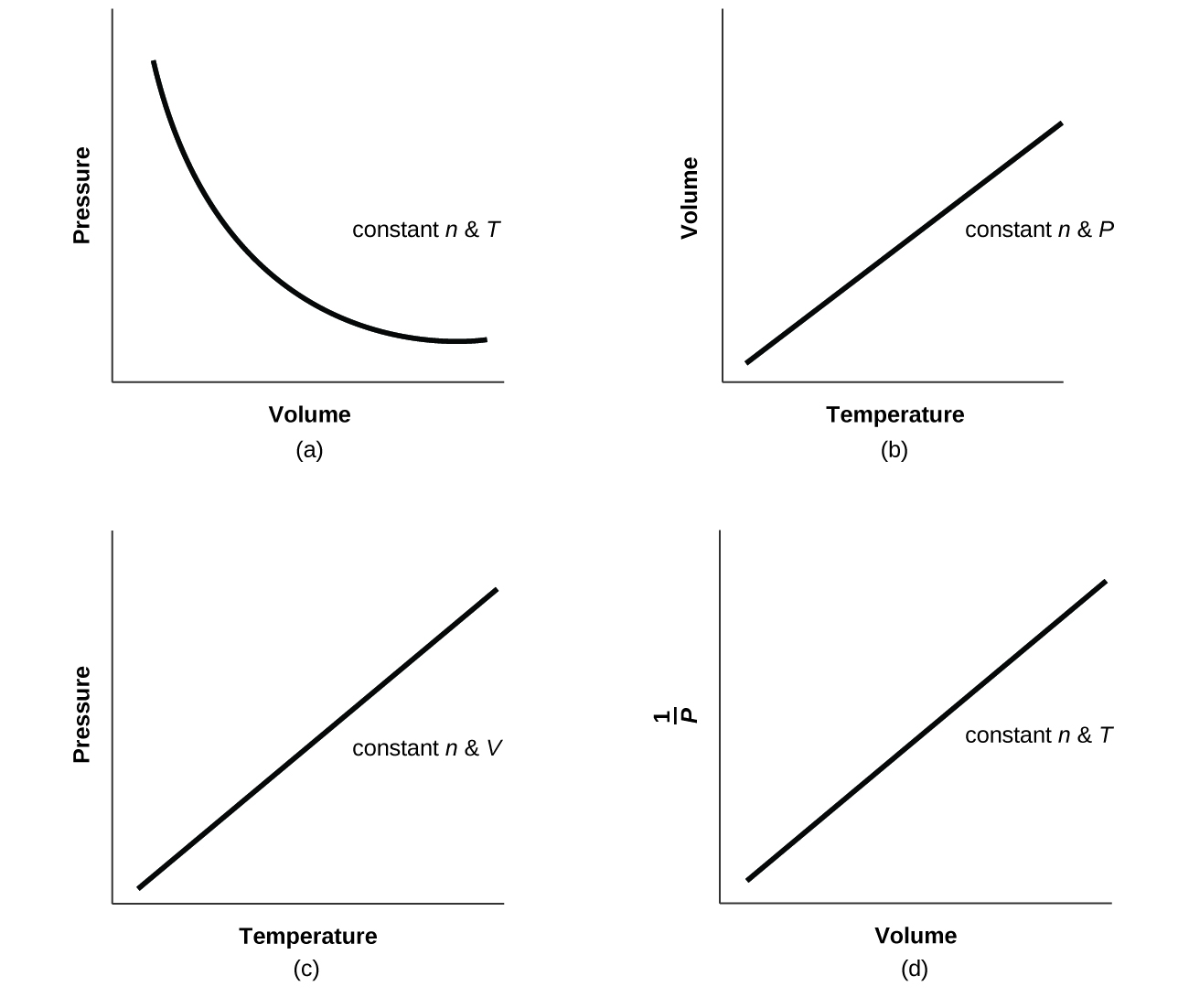
9 2 Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry I

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors

Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors
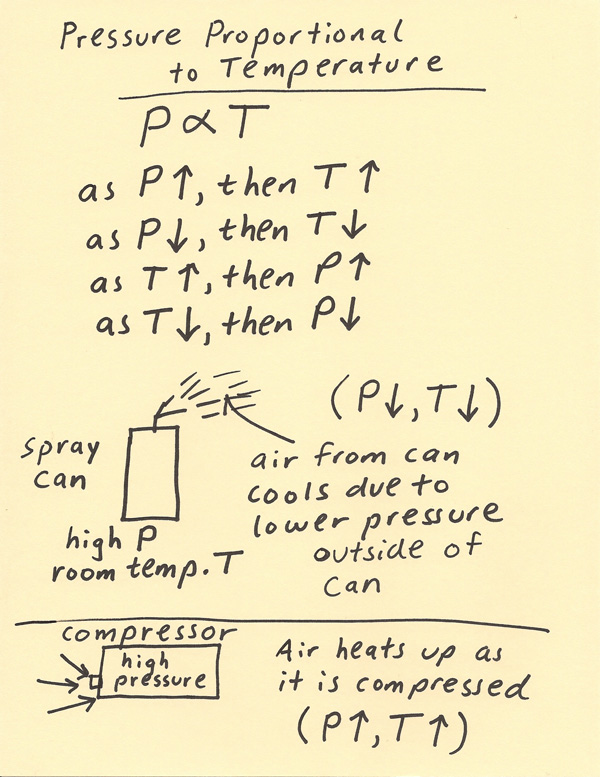
Pressure And Temperature Relationship
Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry For Majors

Gas Laws Boyle S Law Charle S Law Gay Lussac S Law Avogadro S Law
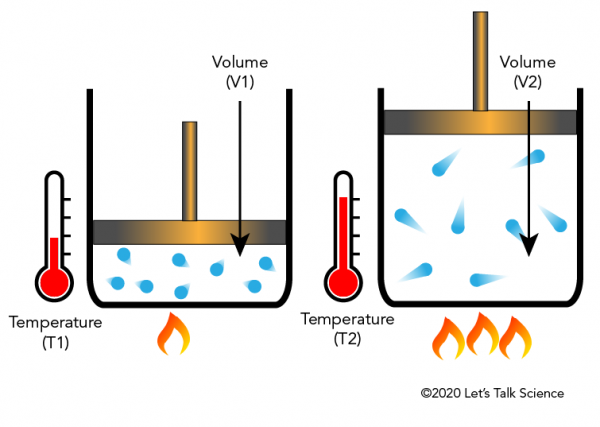
Charles Law And Gay Lussac S Law Let S Talk Science
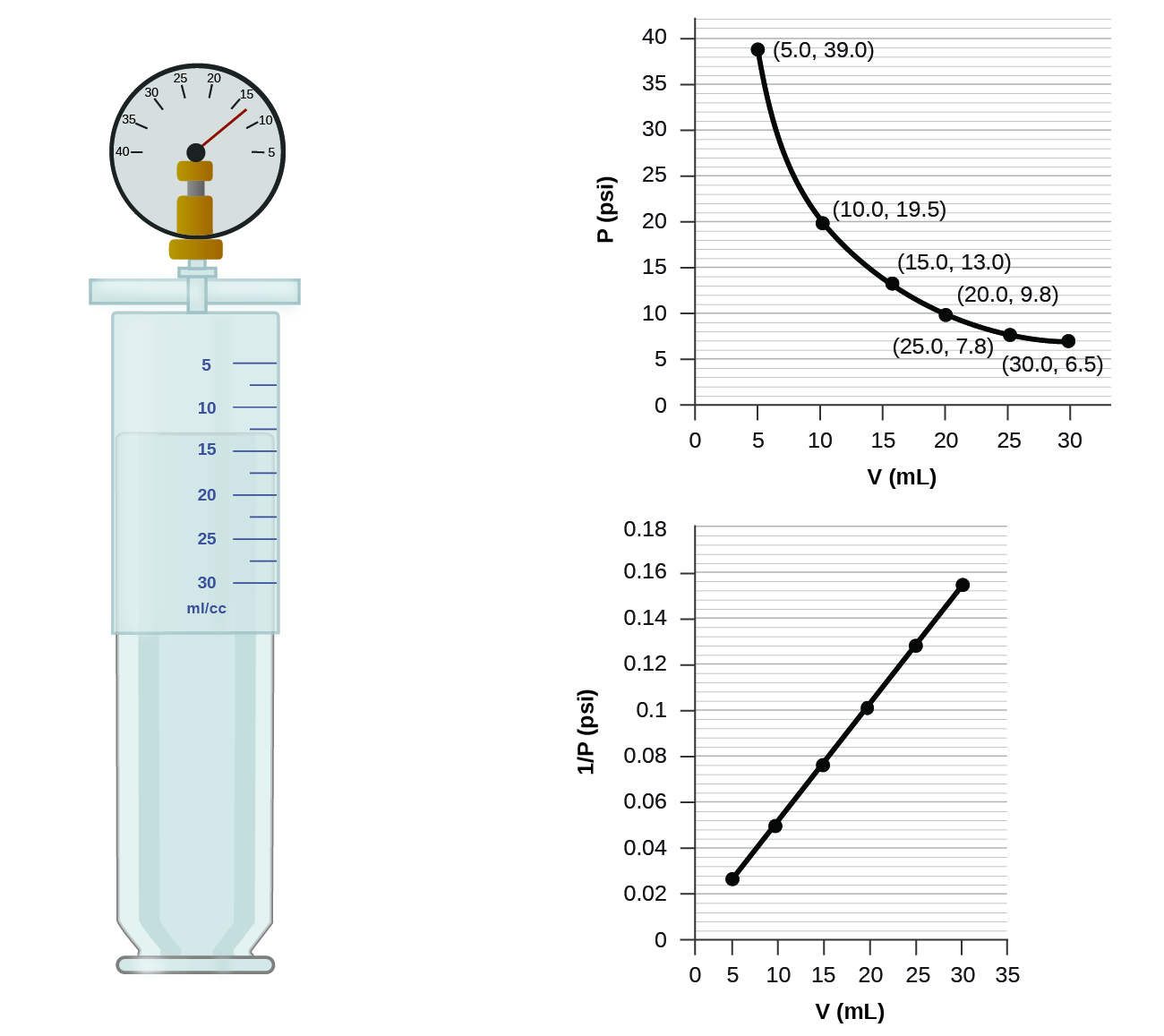
9 2 Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry

Charles S Law Graph Chemistrygod
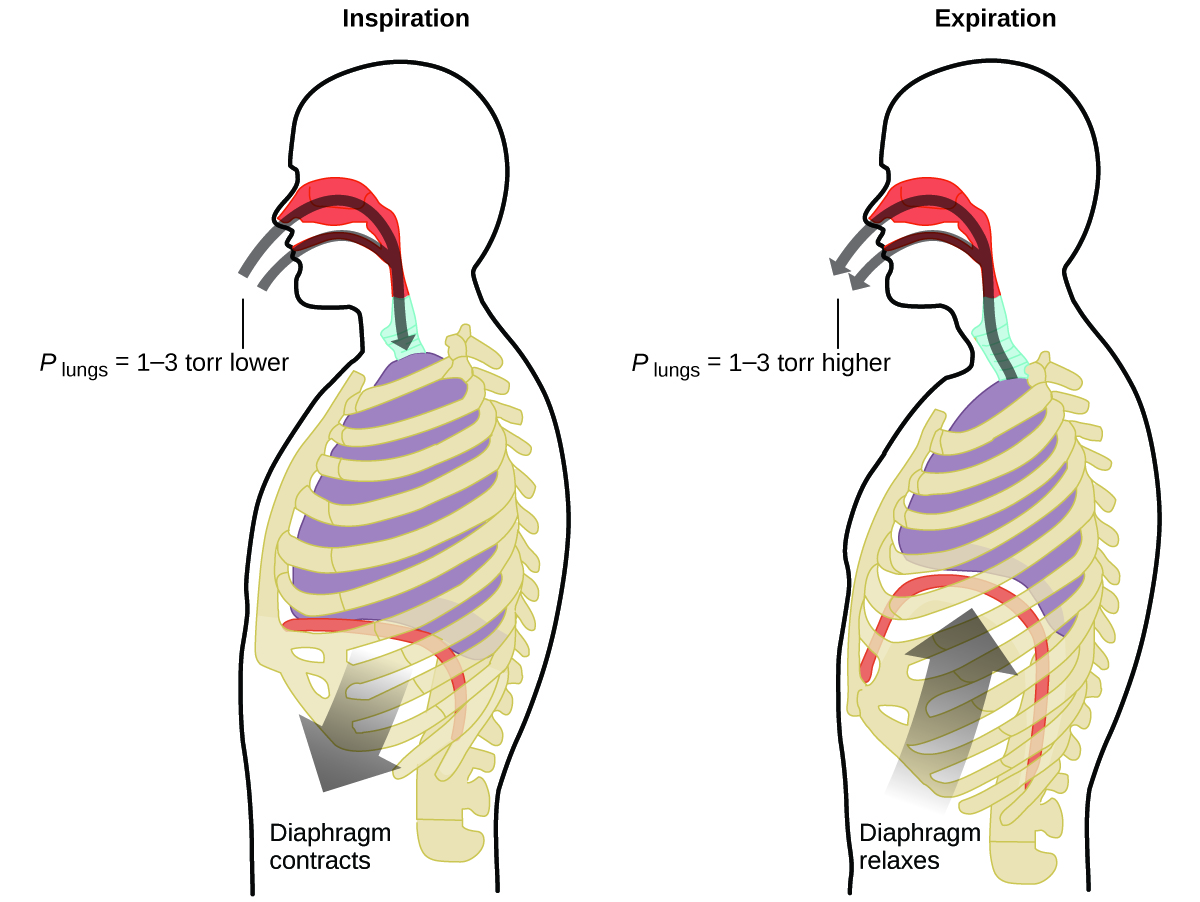
9 2 Relating Pressure Volume Amount And Temperature The Ideal Gas Law Chemistry
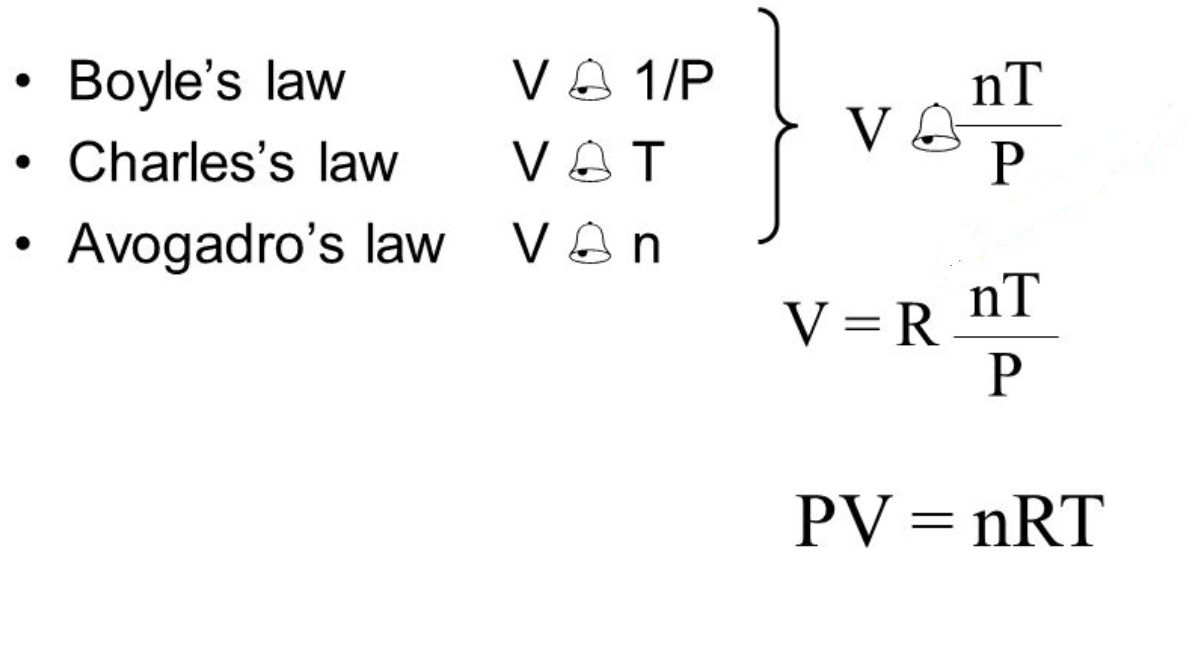


Posting Komentar untuk "How Are Temperature And Volume Related"