Is Calcium Sulfate Aqueous
Compared to other salts like potassium sulfate 110 gL or calcium nitrate 1470 gL the solubility of calcium sulfate seems to be very low 24 gL. National Institutes of Health.

Pdf Modelling Of Calcium Sulphate Solubility In Concentrated Multi Component Sulphate Solutions Semantic Scholar
What is Soluble and Insoluble.

Is calcium sulfate aqueous. Calcium sulfate CaSO4 - PubChem. Department of Health and Human Services. Copper wire and Sulfuric acid react to form aqueous Copper II Sulfate and water and Sulfur Dioxide gas Word Equation.
CaSO4 calcium sulfate is Soluble in water. Follow a rate law of the form76 Tdt ksT0 1 where T is the total calcium or sulfate concentration in solution T0 the solubility value t time in minutes k the crystallization reaction. When looking at the equilibrium between calcium sulfate and its aqueous ions what could be added to solution to promote precipitation of calcium sulfate.
If a little more Ca2 or SO 4 2-is added to a saturated CaSO 4 solution the equilibrium will shift to the left to form solid CaSO 4. So you must either be talking about Na2SO4 or perhaps NaHSO4. The solubility of calcium sulfate dihydrate and calcium sulfate hemihydrate in salt free solutions at 4 atm over the tem- perature range 60 to 125.
National Center for Biotechnology Information. The uptake behavior of AsIIIAsV from aqueous solutions by calcium sulfate whisker CSW dihydrate or anhydrite synthesized through a cooling recrystallization method was explored. A the formation of an aqueous solution by dissolution of impure hemihydrated calcium sulfate at a concentration at most of 130 grams per liter expressed in dissolved CaSO 4 and at a pH of at least 55 b the separation of the aqueous solution.
Firstly NaSO4 is not a stable salt because Na and SO42- sum to a net negative charge. Journal of Chemical Engineering Data 2015 60 9 2559-2566. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent.
Gypsum CaSO 4 2H 2 O bassanite CaSO 4 05H 2 O and anhydrite CaSO 4The intersection of the solubility curves of the different hydrates defines the thermodynamic stability. Aqueous solutions of Calcium Hydroxide and Phosphoric acid react to form aqueous Calcium Phosphate and water. In this paper the heat transfer coefficients of pure water and aqueous solutions of calcium sulfate on.
The addition of ammonium. Effects of Na Ca Mg and Al Chloride Salts on Dissolution and Phase Stability of Calcium Sulfate Dihydrate in Aqueous Solutions at 27815 K to 30815 K. 8600 Rockville Pike Bethesda MD 20894 USA.
In our case the calcium sulfate from the manufacture of industrial phosphoric acid was used as an adsorbent for the removal of metal cations. When considering the equilibrium between copperI carbonate and its aqueous ions what could be added to solution to promote the precipitation of copperI carbonate. Therefore several studies have been carried out for the purpose of removing metal ions from the aqueous solution by materials such as activated carbon kaolinite etc5-8.
Considering that CaCl2 and Na2SO4 is water soluble and C. The solubility of a substance fundamentally depends on the physical and chemical properties of the. A series of batch experiments were conducted to examine the effect of pH reaction time whisker dosage and initial As concentration.
We have investigated the solubility behavior of calcium sulfate dihydrate gypsum CaSO42H2O in aqueous NaCl solutions upon the addition of ammonium- or imidazolium-based ionic liquids ILs viz ethylammonium lactate EAL 1-ethyl-3-methyl imidazolium hydrogen sulfate C2mimHSO4 and 1-butyl-3-methyl imidazolium hydrogen sulfate C4mimHSO4 at 30 C. Either way the answer is the same. Calcium sulfate comprises a group of minerals commonly found in natural and engineered environments.
When CaSO 4 is precipitated from aqueous solutions three crystalline phases can be obtained. National Library of Medicine. The present work is devoted to an.
For this purpose determination of thermodynamic solubility product from reference data and from the composition of possible chemical species existing in equilibrium with solid phase of calcium sulfate dehydrate in aqueous calcium sulfate saturated solutions containing different Ca 2 content is desired. Saturated solutions Any aqueous solution in which the product of the calcium ion concentration and the sulfate ion concentration is about 2410-5 is said to be a saturated CaSO 4 solution. A process for the preparation in an aqueous medium of purified calcium sulfate with high whiteness characterized in that it comprises.
Calcium sulfate has inverse solubility with temperature and showed the potential of deposition in different industries where cooling water is used.

How To Write The Net Ionic Equation For Cacl2 Mgso4 Mgcl2 Caso4 Youtube
Gypsum The Dihydrate Form Of Calcium Sulfate Called

Calcium Definition Properties Compounds Britannica

How To Write The Equation For Caso4 H2o Calcium Sulfate Water Youtube

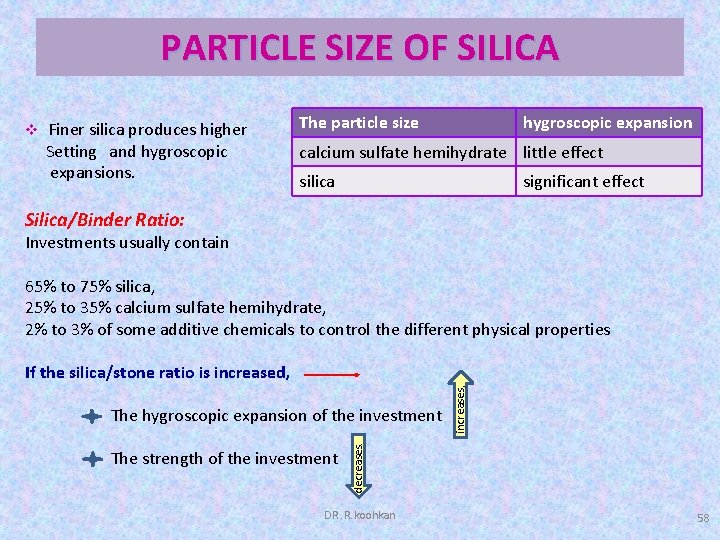
Solubility Of A And B Calcium Sulfate Hemihydrate And Calcium Sulfate Download Scientific Diagram

Phases Of The Calcium Sulphate Water System 6 9 Download Scientific Diagram

Solubility Of Calcium Sulfate Compounds In The Ca So 4 H 2 O System As Download Scientific Diagram

Procedure Method Making Insoluble Salt By Precipitation Reaction From Two Soluble Compounds Apparatus Chemicals Procedures Equations Use Of Barium Sulfate Meal Gcse Chemistry Ks3 Ks4 Science Igcse O Level Revision Notes

Calcium Sulfate An Overview Sciencedirect Topics
Is Caso4 Soluble Or Insoluble In Water Youtube

Calcium Sulfate An Overview Sciencedirect Topics

Calcium Sulfate Caso4 Latest Price Manufacturers Suppliers

Phases Of The Calcium Sulphate Water System 6 9 Download Scientific Diagram

Ppt I F You Add Additional Solid Calcium Sulfate To The Saturated Solution What Will Happen Powerpoint Presentation Id 3197546

Solubility Of A And B Calcium Sulfate Hemihydrate And Calcium Sulfate Download Scientific Diagram

Posting Komentar untuk "Is Calcium Sulfate Aqueous"