What Type Of Bond Does H2 Have
Bonds involving hydrogen can be quite short. H2 is a molecule of two protons sharing 2 electrons in the outermost shell and so of course their bond length will be extremely small.
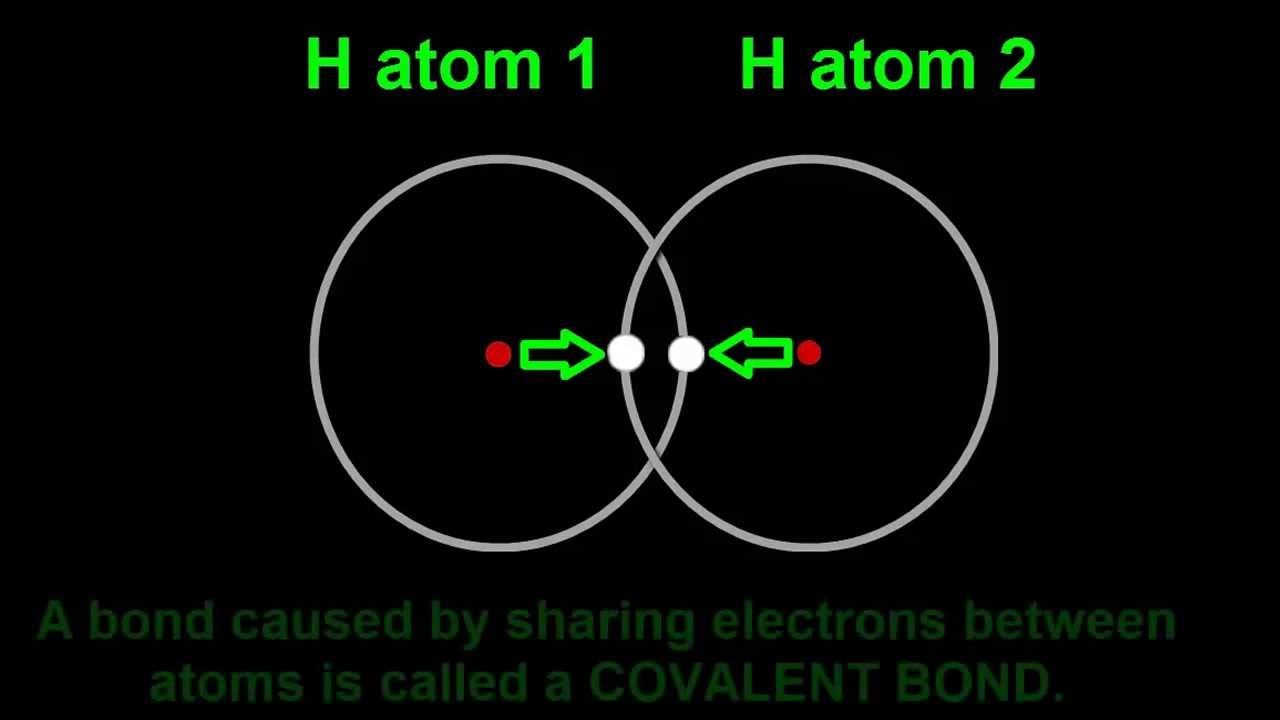
How Two Hydrogen Atoms Join To Become A Hydrogen Molecule H2 Covalent Bonding Molecules Hydrogen Atom
Become a member and.

What type of bond does h2 have. Therefore hydrogen bonding arises in water molecules due to the dipole-dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of. Thanks for reading my answer. When atoms meet in just the right way with just the right needs they will form a single covalent bond an arrangement that holds the two atoms together and gives them lower energy than they would.
Hydrogen bonds form between the δ hydrogen on one HF molecule and a lone pair on the fluorine of another oneThe figure below illustrates this association. Hydrogen atoms in compounds such as H2 and H2O are joined to the molecule with covalent bonds. Typically when the electronegativity difference in a bond is between 05 and 17 the compound is formed by a.
What type of bond is H2. Molecules made of more than one type of covalently bonded nonmetal atoms like carbon dioxide gas CO2 remain nonpolar if they are symmetrical or if their atoms have relatively equal pull. This occurs when two non-metal atoms bond and electron pairs are shared.
Representation of the covalent bond joining two hydrogen atoms in the H2 bond. The H 2 O water molecule is polar with intermolecular dipole-dipole hydrogen bonds. Valence bond theory suggests that H.
The H hydrogen atom is a non metal so the H2 molecule has bonding between two non metals so it is a covalent bond. For example in water molecules H2O hydrogen is covalently bonded to the more electronegative oxygen atom. See full answer below.
What is a dipole-dipole force. Hydrogen bonding is a special type of dipole-dipole attraction between molecules not a covalent bond to a hydrogen atom. H 2 is not a hydrogen bond but is a molecule in which hydrogen is bonded to itself.
It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N O or F atom and another very. Chapter 5 Problem 39Q is solved. Fluorines outer electrons are at the n2 level and the lone pairs represent small highly charged regions of space.
Why is H2 a covalent bond. The electronegativity of hydrogen is approximately 22 while iodine has an electronegativity of around 266. Hydrogen is an example of an extremely simple covalent compound.
For H2O water the type of bonds between atoms are considered covalent molecular. What will be the bond length in H2 molecule. And since it is a bonding between same atoms and there is no difference in the electronegativity between the two atoms is in non polar covalent bond.
What Type of Bond is joining two Hydrogen Atoms Answer. Why does H2 have a short bond. This is where electrons are shared between the atoms in order to fill there valence shells.
A covalent bond is a chemical bond that comes from the sharing of one or more electron pairs between two atoms. 2O describes the bonds as two sigma bonds between the central oxygen atom and the two peripheral hydrogen atoms with oxygen having two lone pairs of electrons. A A single covalent bond holds two hydrogen atoms together in.
It is an example of an intra-molecular force. A hydrogen bond is an intermolecular force IMF that forms a special type of dipole-dipole attraction when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons. Covalent molecules made of only one type of atom like hydrogen gas H2 are nonpolar because the hydrogen atoms share their electrons equally.
H2 makes covalent bond because association is due to sharing of electron H2 is not ionic because An ionic bond is a chemical link between two atoms caused by the electrostatic force between oppositely-charged ions in an ionic compound. Hydrogen iodide is a diatomic molecule formed by a covalent bond. H 2 forms when two hydrogen atoms H are bonded together by a.
As the water molecules attract each other and form bonds water displays properties such as high surface tension and a high heat of vaporization.

Four Covalent Bonds Carbon Has Four Valence Electrons And Here A Valence Of Four Each Hydrogen Atom Has One Vale Covalent Bonding Chemical Bond Ionic Bonding

The Hydrogen Atom Has A Positive Charge Well The Oxygen Atom Has A Negative Charge Making The Compound A Covalent Bond And Covalent Bonding Molecules Chemistry

Basic Chemistry Atoms And Ions Chemistry Chemistry Lessons Chemistry Classroom

H 2 Hydrogen Gas Covalent Bond Bonds In Biology Weak Bonds Hydrogen Bonds Attraction Between And Hydrogen Bond Covalent Bonding Chemistry Basics

The Structure Of A Hydrogen Molecule Covalent Bonding Molecules Gcse Chemistry

Biological Significance Of Hydrogen Bonds In Water Hydrogen Bond Bond Biology Class

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

Hydrogen Bonding Definition Examples And Types Digital Kemistry Hydrogen Bond Bond Molecules

The Atoms In Sugar Are Bounded By What Type Of Bond Ionic Vanderwaals Covelant Hydrogen Online Student Molecules What Type

H2 Covalent Bond Chart Potential Energy Covalent Bonding Chemistry

Hydrogen Bonds Are Intermolecular Forces Despite Their Confusing Title These Are Forced That Exist Betwee Covalent Bonding Intermolecular Force Hydrogen Bond

Learn For Free About Math Art Computer Programming Economics Physics Chemistry Biology Medicine Fina Hydrogen Bond Chemistry Classroom Covalent Bonding

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

How Do Molecular Compounds Bond Example Hydrogen Bond Bond Molecular

Chapter 2 Summary Chemical Bond Teaching Chemistry Science Chemistry

Hydrogen Bond Definition And Examples Hydrogen Bond Bond Covalent Bonding

Hydrogen Bonds Vs Van Der Waals Hydrogen Bond Bond Chemistry
Posting Komentar untuk "What Type Of Bond Does H2 Have"