How Do You Find Protons Electrons And Neutrons
Protons Neutrons and Electrons Worksheet W310 Everett Community College Tutoring Center Student Support Services Program Atomic symbol Atomic number Protons Neutrons Electrons Atomic mass Charge Pb 82 2 34 79 0 24 21 10 9 0 41 35 93 P 15 -3 Rb 85 1 46 106 0 76 114 72. Carbon has 6 protons 6 neutrons and 6 electrons.

How To Find The Number Of Neutrons In An Atom Neutrons Atom Phd Student
If the charge is positive there are more protons than electrons.

How do you find protons electrons and neutrons. A positively-charged ion or cation has more protons than electrons. Finding Protons Neutrons and Electrons. Protons Neutrons and Electrons of all the Elements.
The mass number is the total number of neutrons and protons within the atom. If the atom is neutral the number of electrons will be equal to the number of protons. It also explains the differe.
Hence the original number of electrons and protons in Sodium is equal to 11 but as it has lost one electron the number of electrons number of protons atomic number 10. You can use these numbers to calculate the number of protons neutrons and electrons in an atom. An ion has an unequal number of protons and electrons.
This chemistry video tutorial explains how to calculate the number of protons neutrons and electrons in an atom or in an ion. How do you find the atomic mass. The number of protons neutrons and electrons in an atom can be determined from a set of simple rules.
To find the number of protons electrons and neutrons in an atom just follow these easy steps. KHANTECH Education has brought you lecture of Seemab Ejaz on Chemistry How to find electrons protons and neutrons of an atomChemistry Atom electron. You can simply subtract the atomic number from the mass number in order to find the number of neutrons.
To find the number of neutrons you will need to subtract the atomic number from the atomic mass. The number of protons in the nucleus of the atom is equal to the atomic number Z. How do you find protons neutrons and electrons in an ion.
Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. The proton number is the atomic number of the element while the electron number is the atomic number minus the charge. Atomic Number Z The atomic number is the number of protons in the nucleus of an atom.
Lithium has 3 protons 4 neutrons and 3 electrons. A neutral atom has the same number of protons and electrons. How do you determine the number of protons in an element.
Look at the bottom of the box and yes. Subtract atomoc mass from the number of protons. Hydrogen has 1 proton 0 neutron and 1 electron.
Look at the number of protons and that will be the electrons. A neutral atom has the same number of protons and electrons charges cancel each other out. In a balanced atom the number of electrons equals the number of protons.
The number on the upper left corner is the mass number which is equal to the neutrons and protons added together. The number on the bottom left corner is the atomic number which tells you the number of protons. How do you find the neutron.
Helium has 2 protons 2 neutrons and 2 electrons. How do you find the atomic number. Step 1 - Gather Information The first thing you will need to do is find some information about your element.
The number of electrons in a neutral atom is equal to the number of protons. In an unbalanced atom the number of electrons equals the number of protons plus the opposite of the ion charge. Determining Protons Neutrons and Electrons of Atoms and Ions.
For boron 11 atomic mass 5 atomic number 6 neutrons. For neutral atoms the electron number is the same as the atomic number. Protons Neutrons and Electrons.
To the nearest tenth. It is listed on the periodic table for each element. Similarly the number of neutrons in Na Mass Number Atomic Number 23 10 13.
Lastly the charge is on the upper right corner. No two elements have the same atomic number or the same number of protons so the atomic number identifies the element. Number of protons number of electrons atomic number Number of neutrons mass number.
You need the atomic number to find the amount of protons andor electrons unless you have the amount of neutrons and the atomic mass in which case you can simply subtract the amount of neutrons from the atomic mass leaving the amount of protons. Boron has 5 protons 6 neutrons and 5 electrons. Beryllium has 4 protons 5 neutrons and 4 electrons.
Calculate the number of neutrons by subtracting the atomic number from the mass number. This number is the atomic number of the element.

The Atom Chemistry Is My Jam Atom Neutrons Electron Configuration

How To Calculate Atomic Mass Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets

How To Find The Number Of Protons Neutrons And Electrons Neutrons Electrons Protons

Atom Ape Man Atomic Mnemonic For Protons Neutrons Amp Electrons From The Sci Lander On Teachersnotebook Com Chemistry Worksheets Mnemonics Matter Science

This Guided Inquiry Lesson Enables Students To Construct Their Own Understanding Of How To Determine T Teaching Chemistry Chemistry Education Science Chemistry

Online Activity Determining How Many Protons Electrons And Neutrons In An Atom Based On The Periodic Science Chemistry Physical Science Homeschool Science

How To Read The Periodic Table High School Chemistry Electrons Electron Configuration

How To Find The Number Of Protons Neutrons And Electrons Protons Neutrons Electrons

What Are The Characteristics Of Electron Proton And Neutron A Plus Topper Https Www Aplustopper Com Characteristics Electron Protons Neutrons Electrons

Calculating Parts Of An Atom Practice Worksheet Bundle Practices Worksheets Chemistry Worksheets Worksheets

Protons Neutrons Electrons And Isotopes Youtube Protons Neutrons Electrons
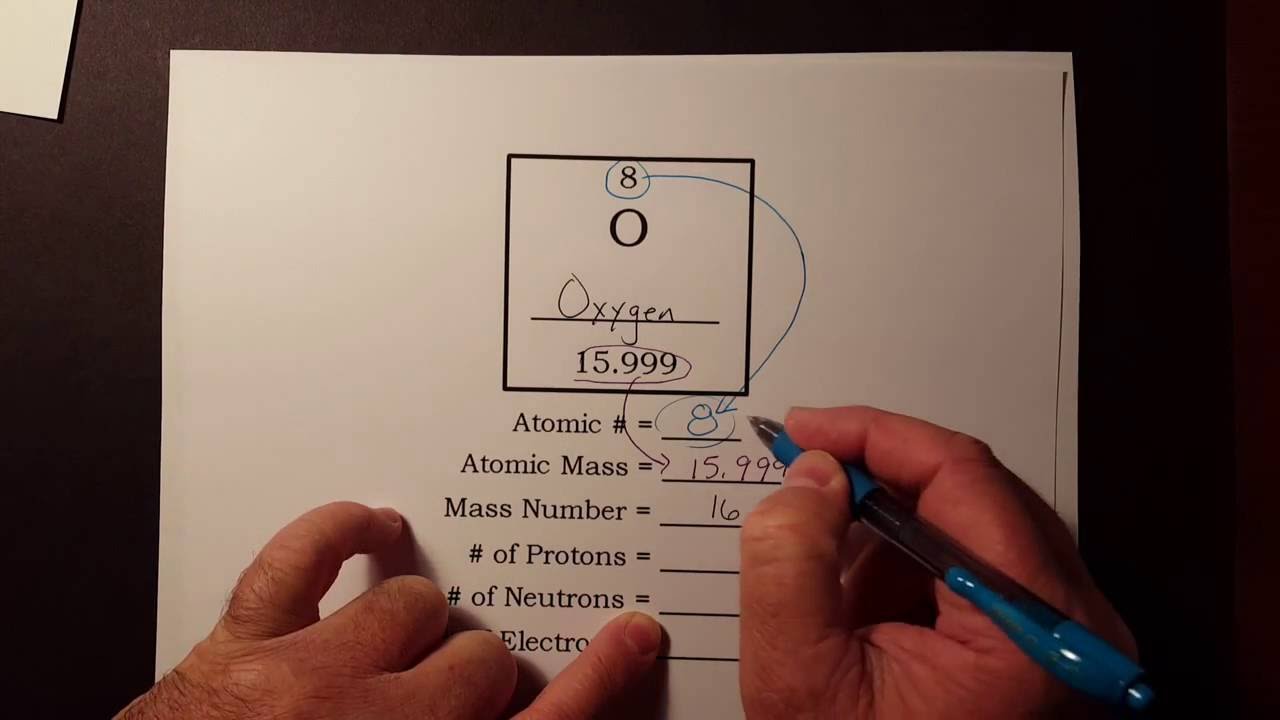
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Protons Neutrons Electrons

Quiz Element Math Calculating Number Of Protons Neutrons Electrons Counting Atoms Worksheet Printable Worksheets Chemistry Worksheets

Determining Protons Neutrons And Electrons Atoms And Ions Electrons Neutrons Protons

How To Find The Number Of Protons Neutrons And Electrons Neutrons Protons Electrons

Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic Proton Neutron Electron Protons Neutrons

Calculating Parts Of An Atom Practice Worksheet 4 Practices Worksheets Chemistry Worksheets Worksheets
Posting Komentar untuk "How Do You Find Protons Electrons And Neutrons"