How To Know The Number Of Protons Electrons And Neutrons
They are both equal thus making the atom have a neutral charge. Periodic Table Basics Learn how to use information from the periodic table to find the number of protons neutrons and electrons of an element.
What Are The Characteristics Of Electron Proton And Neutron A Plus Topper Https Www Aplustopper Com Characteristics Electron Protons Neutrons Electrons
The number of protons is equal to the number of electrons unless theres an ion superscript listed after the element.

How to know the number of protons electrons and neutrons. That number is equal to the number of protons. You cant really get the number of neutrons since that may vary in one and the same element most elements have different isotopes. A neutral atom has the same number of protons and electrons charges cancel each other out.
The relative atomic mass is the number of protons AND neutrons. The number of neutrons is equal to the difference between the mass number of the atom M and the atomic number Z. However it is possible to remove electrons and not change the identity of an element.
For a neutral atom the number of protons is exactly equal to the number of electrons. A positively-charged ion or cation has more protons than electrons. The proton number is the atomic number of the element while the electron number is the atomic number minus the charge.
This is the heart of mass spectrometry although one can use time-of-flight techniques rather than or in addition to magnetic separation. How many electrons protons and neutrons does nitrogen Sep 03 2021 Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. You can however get the average by looking up the properties of each element.
You can simply subtract the atomic number from the mass number in order to find the number of neutrons. The atomic number is the number of protons OR electrons. If the charge is positive there are more protons than electrons.
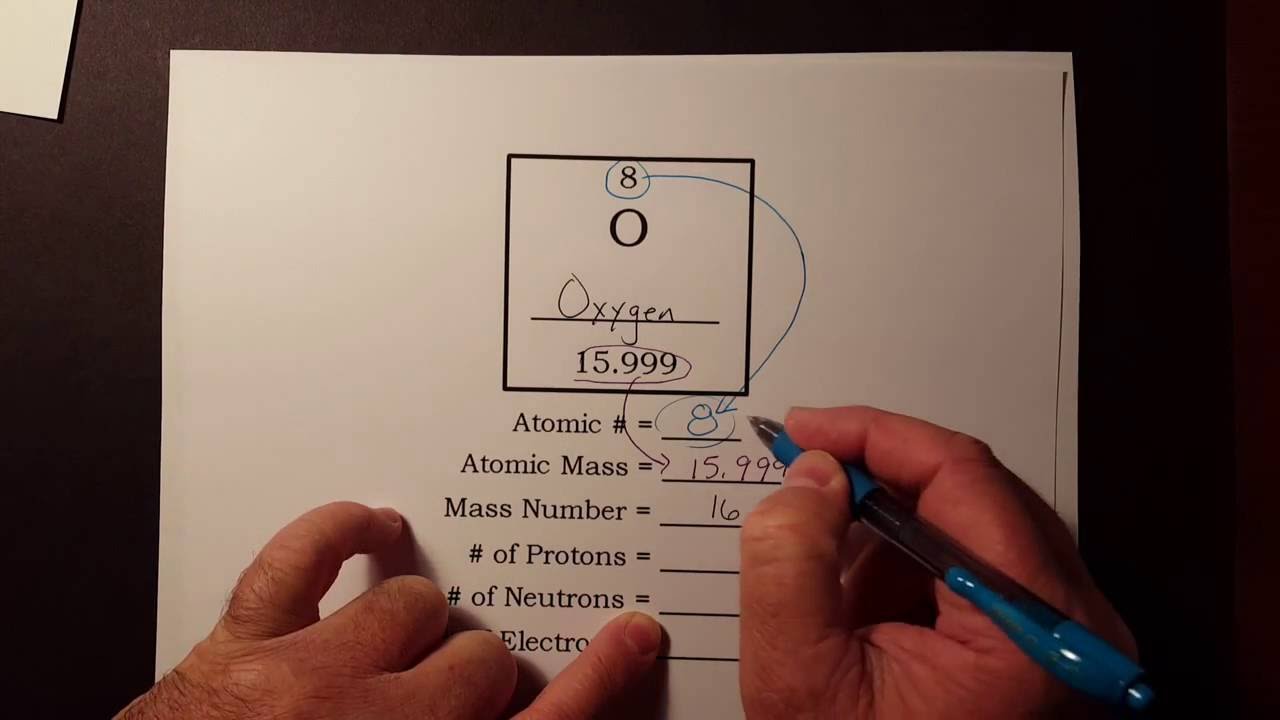
The number of electrons in an element can change. If the atom is neutral the number of electrons will be equal to the number. The easiest way to find the number of protons neutrons and electrons for an element is to look at the elements atomic number on the periodic table.
The atomic number number at the top is the amount of protons and the amount of electrons. You just need to know a couple tricks to always be able to find the number of protons neutrons and electrons. The number of protons in an atom is the same as.
The number on the bottom left corner is the atomic number which tells you the number of protons. Whichever you know you subtract from the atomic mass. This number is the atomic number of the element.
A neutral atom has the same number of protons and electrons. So the number of electrons is the same as the atomic number. Which is often the case.
You subtract the atomic number from the atomic mass to find the number of neutrons alone. An ion has an unequal number of protons and electrons. The mass number of the atom M is equal to the sum of the number of protons and neutrons in the nucleus.
So if an element has an atomic number of 5 you know that it has 5 protons and 5 electrons. Protons carry a positive electrical change while electrons are negatively charged and neutrons are neutral. From the magnet deflections one can get a charge-to-mass ratios and get back to number of protons charge on a fully stripped ion and number of neutrons from number of protons and the mass.
The atomic mass number at the bottom is the amount of protons and neutrons added together. How many electrons are there. The number of electrons is equal to the number of protons assuming the molecules are neutral.
Lastly the charge is on the upper right corner. The number on the upper left corner is the mass number which is equal to the neutrons and protons added together. You know that nitrogen-14 has 7.
How To Find The Number Of Protons Neutrons And Electrons Protons Electrons Neutrons
Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic Proton Neutron Electron Protons Neutrons
How To Find The Number Of Protons Neutrons And Electrons Protons Neutrons Electrons
Counting Atoms Protons Neutrons Electrons Worksheet Text Features Worksheet Counting Atoms Neutrons
How To Find The Number Of Protons Neutrons And Electrons Neutrons Protons Electrons
Protons Neutrons And Electrons Practice Worksheet Proton Neutron Electron Practices Worksheets Protons
Quiz Element Math Calculating Number Of Protons Neutrons Electrons Counting Atoms Worksheet Printable Worksheets Chemistry Worksheets
How To Find The Number Of Protons Neutrons And Electrons Neutrons Protons Proton Neutron Electron
Basic Parts Of The Atom Protons Neutrons Electrons Nucleus Atom Matter Science Protons
Calculating Parts Of An Atom Practice Worksheet Bundle Practices Worksheets Chemistry Worksheets Worksheets
Determining Protons Neutrons And Electrons Atoms And Ions Electrons Neutrons Protons
Calculating Parts Of An Atom Practice Worksheet 4 Practices Worksheets Chemistry Worksheets Worksheets
How To Find The Number Of Neutrons In An Atom Neutrons Atom Phd Student
Atom Ape Man Atomic Mnemonic For Protons Neutrons Amp Electrons From The Sci Lander On Teachersnotebook Com Chemistry Worksheets Mnemonics Matter Science
Online Activity Determining How Many Protons Electrons And Neutrons In An Atom Based On The Periodic Science Chemistry Physical Science Homeschool Science
Protons Neutrons Electrons And Isotopes Youtube Protons Neutrons Electrons
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Protons Neutrons Electrons
Posting Komentar untuk "How To Know The Number Of Protons Electrons And Neutrons"